Jose Luis Cabrera Alarcón, PhD

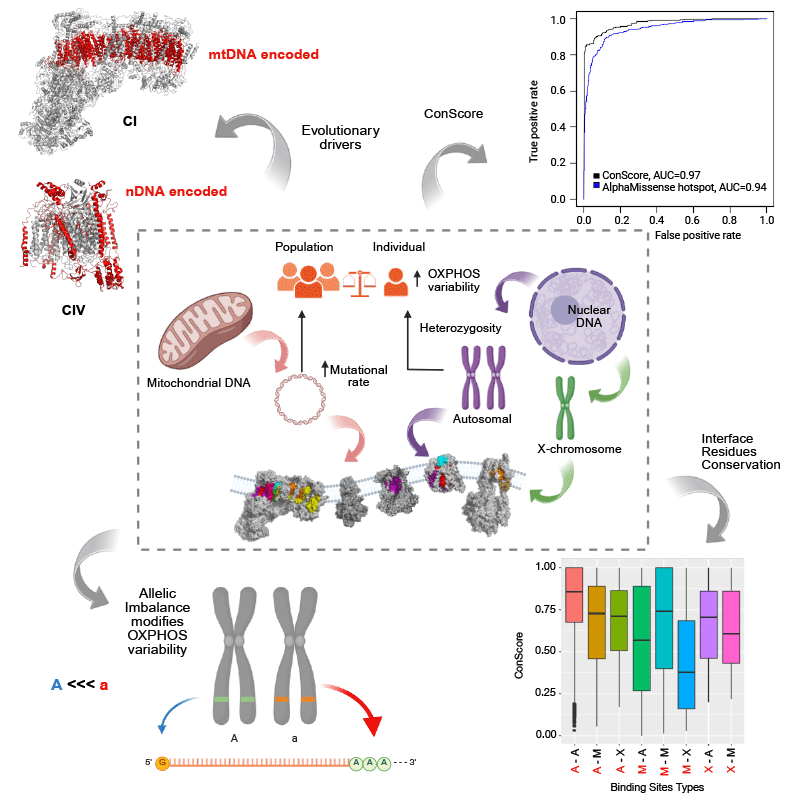
Over six years as a postdoctoral researcher in the GENOXPHOS group, I have explored the genetics of OxPhos components, focusing on population-level variability, evolutionary conservation, and structural effects to predict mitonuclear incompatibilities. One aspect of this research involves mapping, at the structural level, regions in mitochondrial subunits where pathogenic variants can be compensated, as well as identifying areas where such compensation is more challenging.
Pablo Hernansanz Agustín, PhD
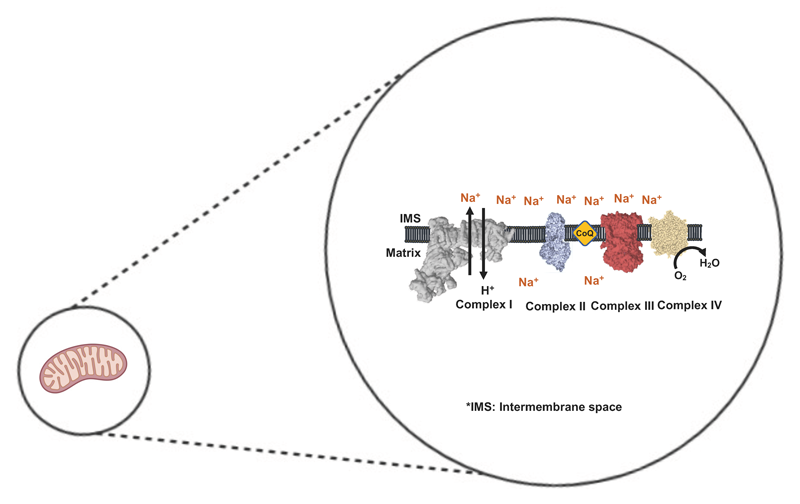
 My research is focused on the role of Na+ in the regulation the oxidative phosphorylation (OXPHOS) system related to bioenergetics, electron flux and the production of reactive oxygen species (ROS), as well as the relationship of all of them with Parkinson’s and mitochondrial diseases. We have recently discovered that Na+ establishes a gradient across the inner mitochondrial membrane, constituting up to 50% of the membrane potential in coupled respiring mitochondria through the action of a a Na+/H+ exchanger (NHE) function by complex I (CI). Mutations in this complex, only affecting only the NHE, are related to neurodegenerative diseases. We are currently exploring how this activity explains a broad spectrum of (patho)physiological conditions and trying to understand the implications of the de-regulation of this activity in human health.
My research is focused on the role of Na+ in the regulation the oxidative phosphorylation (OXPHOS) system related to bioenergetics, electron flux and the production of reactive oxygen species (ROS), as well as the relationship of all of them with Parkinson’s and mitochondrial diseases. We have recently discovered that Na+ establishes a gradient across the inner mitochondrial membrane, constituting up to 50% of the membrane potential in coupled respiring mitochondria through the action of a a Na+/H+ exchanger (NHE) function by complex I (CI). Mutations in this complex, only affecting only the NHE, are related to neurodegenerative diseases. We are currently exploring how this activity explains a broad spectrum of (patho)physiological conditions and trying to understand the implications of the de-regulation of this activity in human health.
Marta Pérez-Hernández Durán, PhD

After defending my PhD at the University Complutense of Madrid (Spain) in cardiac electrophysiology, I did a first postdoctoral fellowship at New York University (U.S.) studying the physiology of cardiomyocytes in the context of arrhythmogenic right ventricular cardiomyopathy. I then joined the GENOXPHOS group to study the role of the tyrosine kinase Fgr in the heart.
Previous studies in our laboratory have shown that Fgr, in the mitochondria, upon stress, phosphorylates complex II of the electron transport chain. This leads to a change in the metabolism of the cell, which ultimately leads to inflammation.
My project studies the cardioprotective effect of Fgr inhibition to reduce cardiac inflammation. Our results could then serve as a foundation to explore treatment of other cardiac conditions in which an inflammatory component plays an important role such as myocardial infarction, atherosclerosis or arrhythmogenic right ventricular cardiomyopathy.
In collaboration with Nostrum, we are developing molecules to specifically target and inhibit Fgr.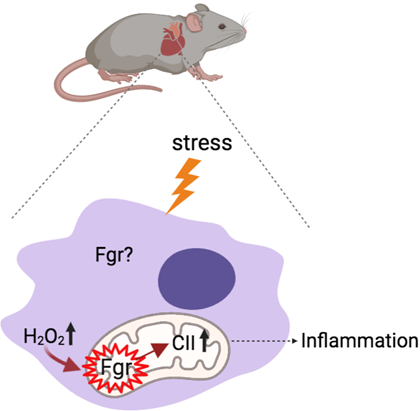
María Concepción Jiménez Gómez, PhD
 After obtaining a doctorate in Molecular Biology from the UAM, I began working in the Research and Development department of a Spanish biotechnology company. In 2010, I decided to refocus my professional career and joined the Genoxphos group, led by Dr. Enríquez at CNIC. Over the last few years, I have managed the group's projects, as well as the translational development initiatives of basic research discoveries. The Genoxphos group collaborates on projects with leaders in the pharmaceutical industry, including Astrazeneca and Minovia Therapeutics. The continuous training of the team is essential for the Genoxphos group. I have completed two master's degrees in management and administration to make better use of the group's resources. Currently, I am studying psychology, to carry out comparative psychology studies in research projects.
After obtaining a doctorate in Molecular Biology from the UAM, I began working in the Research and Development department of a Spanish biotechnology company. In 2010, I decided to refocus my professional career and joined the Genoxphos group, led by Dr. Enríquez at CNIC. Over the last few years, I have managed the group's projects, as well as the translational development initiatives of basic research discoveries. The Genoxphos group collaborates on projects with leaders in the pharmaceutical industry, including Astrazeneca and Minovia Therapeutics. The continuous training of the team is essential for the Genoxphos group. I have completed two master's degrees in management and administration to make better use of the group's resources. Currently, I am studying psychology, to carry out comparative psychology studies in research projects.
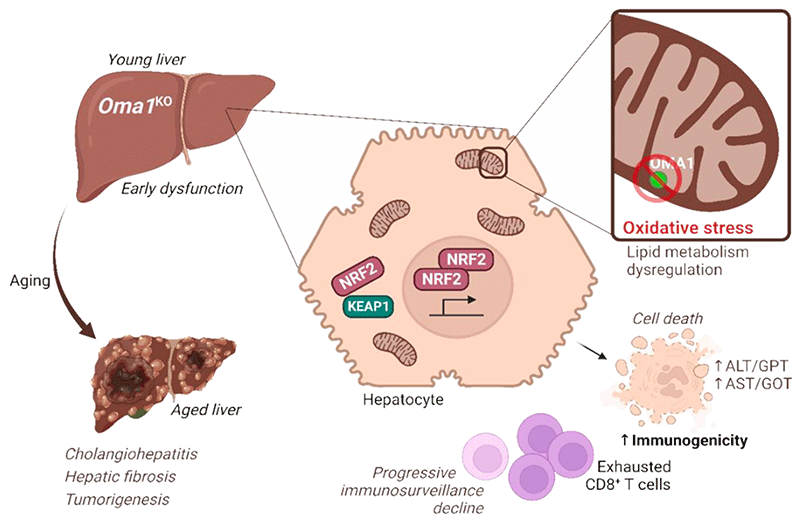
On the other hand, at the research level, I work on the role of the mitochondrial metalloprotease OMA1, as a central integrator of stress in the cell, together with Dr. Yolanda Martí.
OMA1 deletion has been reported to be protective in several pathological contexts, including heart failure. However, recent evidence indicates that OMA1 deletion may be relevant for certain pathologies. Analyzing aged mice with OMA1 deletion (Oma1KO mice), we have observed an increase in mortality as well as the incidence of primary liver tumors, compared to control mice (Oma1WT mice).
Before tumor, young Oma1KO mice already show early liver damage and increased NRF2-dependent oxidative stress, leading to increased cell death in the liver. Over time, Oma1KO CD8+ T cells become progressively depleted, and the early liver damage observed in Oma1KO progresses to chronic liver inflammation and liver fibrosis. The dysfunction observed in Oma1KO animals originates in hepatocytes, indicating that OMA1 protects from chronic liver disease and tumorigenesis by controlling liver immunogenicity.
Raquel Justo Méndez
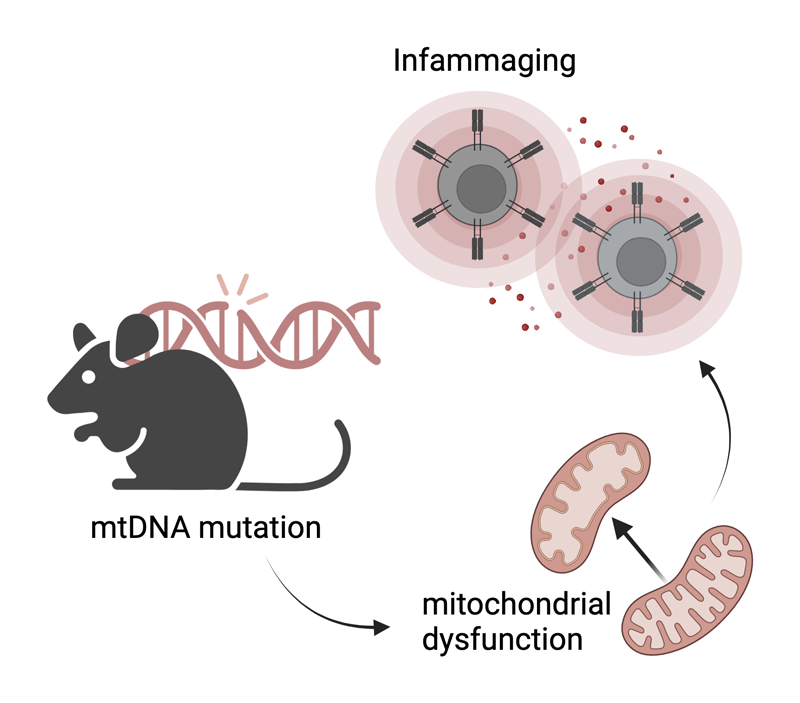
I am biologist (Universidad Autónoma de Madrid) currently performing my phD in Molecular Biomedicine under the supervision of José Antonio Enríquez and Ana Victoria Lechuga-Vieco in which we are evaluating the impact of mito-nuclear crosstalk during hematopoiesis under conditions of normal vs. high mitochondrial variability. Our study involves immune, metabolic, and mitochondrial profiling, focusing on aspects such as ROS management, organization of respiratory complexes, and fuel preference.
By using a mouse model deficient in the proofreading capacity of the mitochondrial DNA polymerase we shed light on how immune cells contribute to inflammaging, frailty and age-related phenotypes, emphasizing the significance of mitochondrial quality control and translation mechanisms.
Carmen Morales Vidal
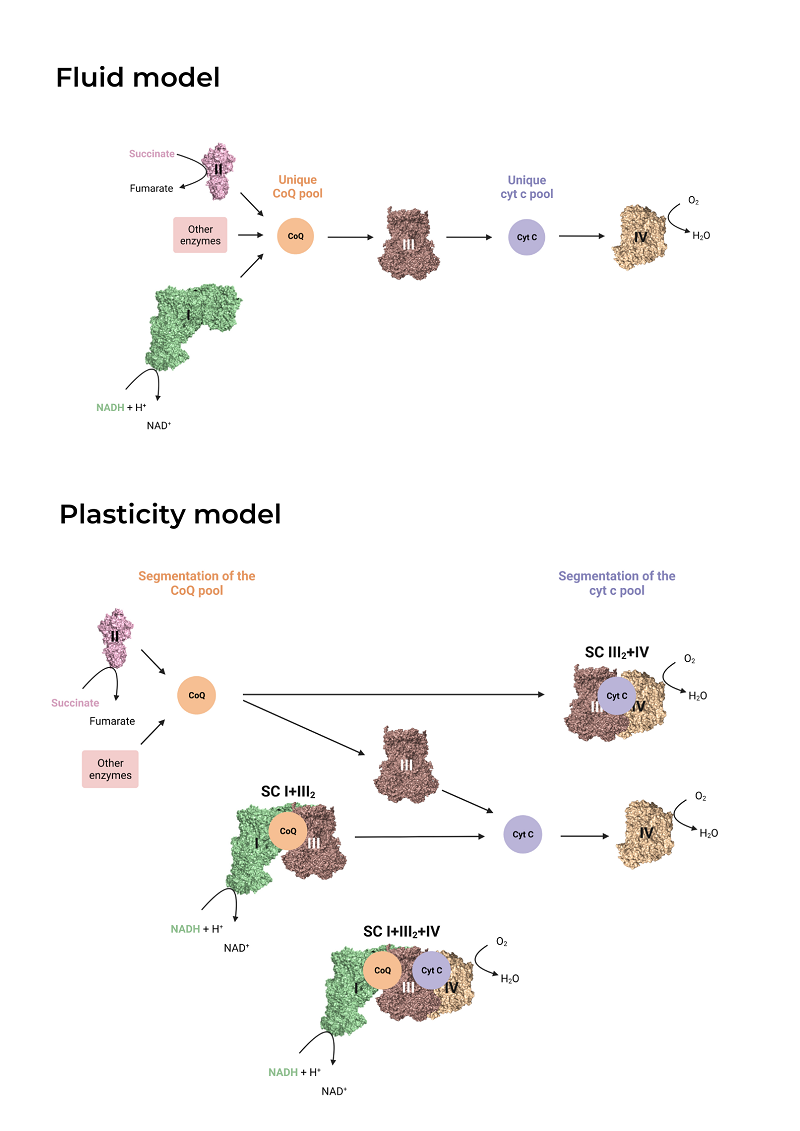
 After graduating in Biochemistry and Biomedical Sciences at the University of Valencia in 2020, I joined the Genoxphos group as a Master’s student and finally as a predoctoral researcher at the end of 2021. My thesis project seeks to understand the reason behind the superassembly of mitochondrial complexes I (CI) and III (CIII), an interaction that is highly conserved in nature, from yeast to mammals, and that gives rise to supercomplexes such as I+III2 and I+III2+IV. Several functions have been proposed for this association, including the channeling of the substrate, ubiquinone, from one complex to the other and the reduction of reactive oxygen species production. In order to further study the role of the I+III2 interaction in the functioning of the respiratory chain and mitochondrial and cellular metabolism, I am currently generating cell lines in which the interaction between CI and CIII is disrupted. This work material will be the starting point to study the importance of the I+III2 interaction in living beings.
After graduating in Biochemistry and Biomedical Sciences at the University of Valencia in 2020, I joined the Genoxphos group as a Master’s student and finally as a predoctoral researcher at the end of 2021. My thesis project seeks to understand the reason behind the superassembly of mitochondrial complexes I (CI) and III (CIII), an interaction that is highly conserved in nature, from yeast to mammals, and that gives rise to supercomplexes such as I+III2 and I+III2+IV. Several functions have been proposed for this association, including the channeling of the substrate, ubiquinone, from one complex to the other and the reduction of reactive oxygen species production. In order to further study the role of the I+III2 interaction in the functioning of the respiratory chain and mitochondrial and cellular metabolism, I am currently generating cell lines in which the interaction between CI and CIII is disrupted. This work material will be the starting point to study the importance of the I+III2 interaction in living beings.
Macarena de Andrés Laguillo
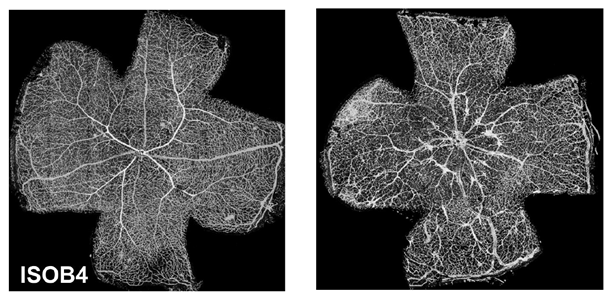
 VHL disease, a genetic disorder, manifests in vascular tumors called hemangioblastomas, primarily found in the retinas and brains of patients. Our research delves into the molecular mechanisms driving their development. Employing a mouse genetic model, we aim to unravel how different cell types, both endothelial and non-endothelial, contribute to hemangioblastoma formation. We've discovered that VHL-deficient cells, with their altered metabolism, influence the behavior of neighboring cells. Additionally, we're investigating the role of hypoxia-inducible factor 2 (HIF2) in this process.
VHL disease, a genetic disorder, manifests in vascular tumors called hemangioblastomas, primarily found in the retinas and brains of patients. Our research delves into the molecular mechanisms driving their development. Employing a mouse genetic model, we aim to unravel how different cell types, both endothelial and non-endothelial, contribute to hemangioblastoma formation. We've discovered that VHL-deficient cells, with their altered metabolism, influence the behavior of neighboring cells. Additionally, we're investigating the role of hypoxia-inducible factor 2 (HIF2) in this process.
Raquel Martínez de Mena, PhD
 After obtaining my PhD in Science from the Universidad Autónoma de Madrid and several years of research focused on brown adipose tissue and thyroid hormones at the IIB Alberto Sols in Madrid, I joined Dr. José Antonio Enríquez's team.
After obtaining my PhD in Science from the Universidad Autónoma de Madrid and several years of research focused on brown adipose tissue and thyroid hormones at the IIB Alberto Sols in Madrid, I joined Dr. José Antonio Enríquez's team.
Over the last 7 years, I have contributed my expertise in biochemical and molecular biology techniques, focusing on Functional Genetics of the Oxidative Phosphorylation System.
I provide scientific and technical support and actively participate in several projects within the group.
María del Mar Muñoz Hernández
 I joined the GENOXPHOS group after finishing my training as a laboratory technician, and I am currently completing my academic training with a Biology degree at Universidad Autónoma de Madrid.
I joined the GENOXPHOS group after finishing my training as a laboratory technician, and I am currently completing my academic training with a Biology degree at Universidad Autónoma de Madrid.
In the group I am responsible for various aspects of research, focusing on experimental animal models, including breeding and genetic characterization of these. I also have special training in surgery (especially cardiac) and metabolic and behavioral analysis in aging.
Eva Raquel Martínez

As a laboratory technician, I am responsible for conducting experiments, analyzing samples, and performing tests in a laboratory. My role often involves using various scientific instruments and ensuring accurate recording of data for research or diagnostic purposes.
These techniques range from DNA genotyping to the treatment of tissue samples with fine diagnostics.
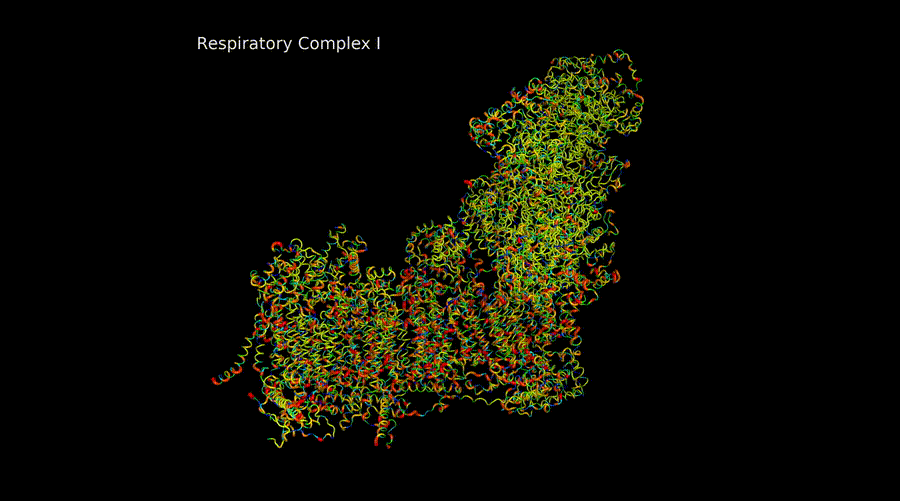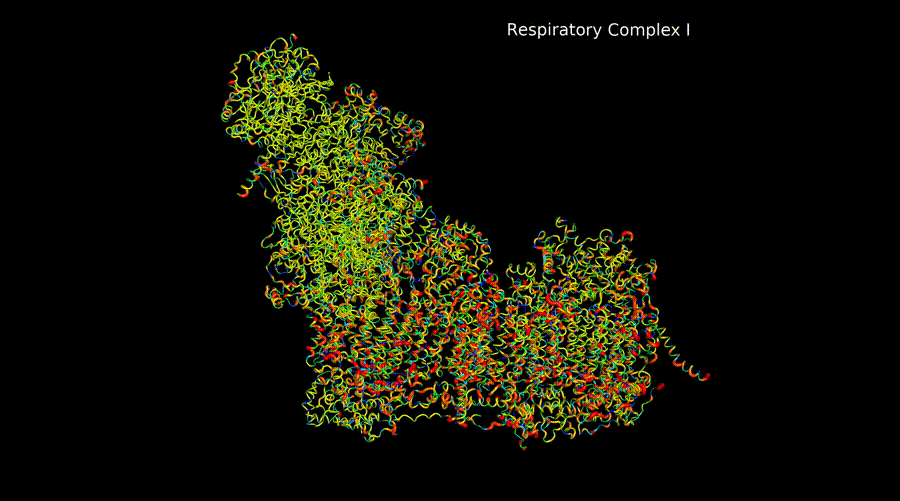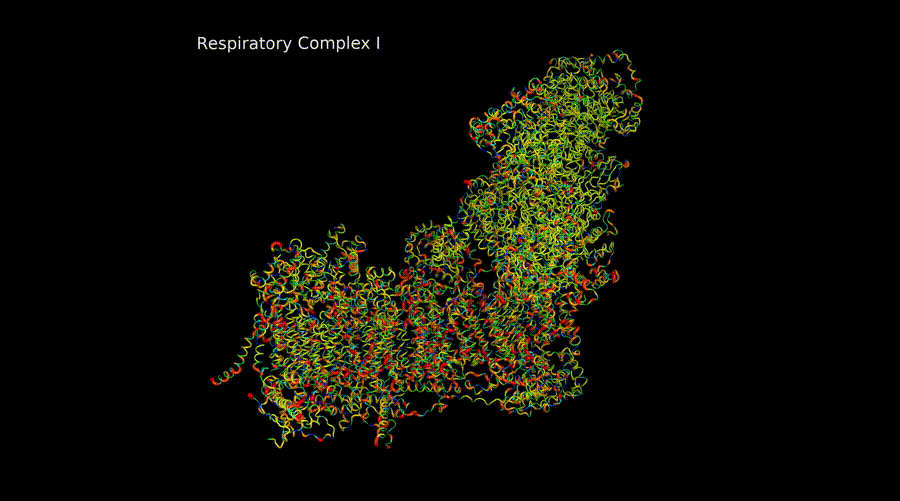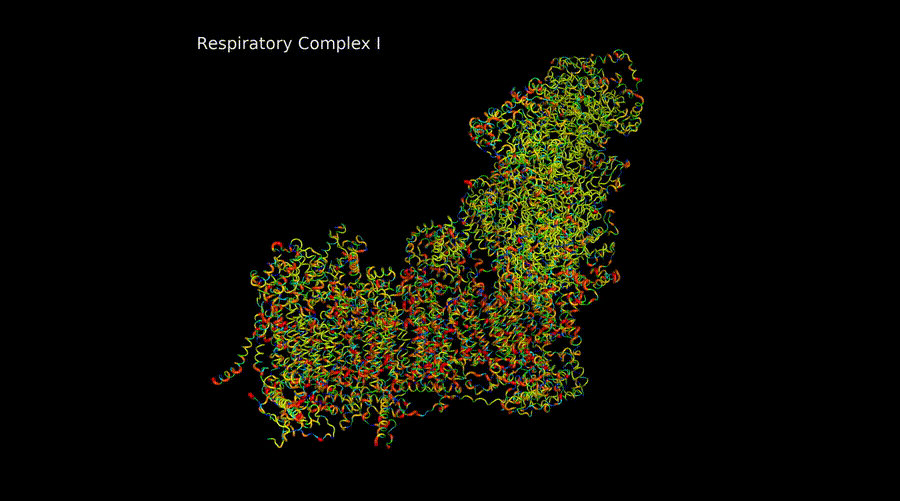
Marina Rosa Moreno
 The electron transport chain complexes are intricate machines that must assemble correctly subunits encoded both in the mitchondrial DNA and in the nuclear DNA for its correct functioning. They also present duplicated subunits, subunits encoded by paralogue genes, etc. And are able to form supercomplexes to cover metabolic demands of the cell.
The electron transport chain complexes are intricate machines that must assemble correctly subunits encoded both in the mitchondrial DNA and in the nuclear DNA for its correct functioning. They also present duplicated subunits, subunits encoded by paralogue genes, etc. And are able to form supercomplexes to cover metabolic demands of the cell.
My project in the lab is based on the structural study of these complexes both by in silico computational modeling and by CryoEM. These models can be used together with databases genomic information to create pathogenicity models and perform evolution studies.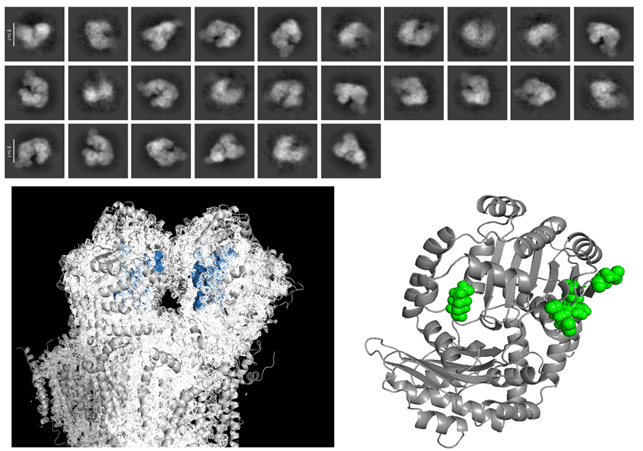
Paula Fernández-Montes Díaz

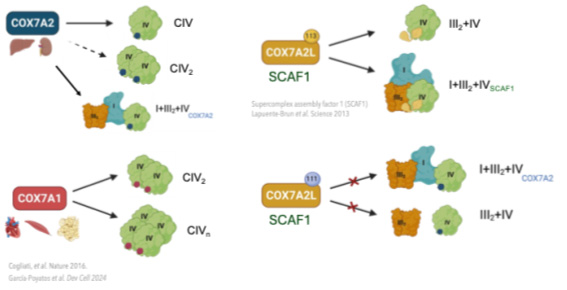
After graduating in Biology at the Universidad Autónoma de Madrid, I joined the GENOXPHOS group as a master student and finally as a predoctoral researcher. In my third year, I study the role of alternative isoforms of complex IV, specifically the Cox7a family. Complex IV (cytochrome oxidase c) is the last complex in the electron transport chain and undergoes a high level of regulation. The COX7A family is formed by different isoforms: COX7A1, COX7A2 and COX7A2L or SCAF1 and they are interchangeable. The alternative Cox7a subunits confer to complex IV different supramolecular organization, appearing in monomeric, dimer, multimer and super-assembled with other complexes forming the Q-respirasome and the N-respirasome (Cogliati and Calvo, et al. Nature 2016).
Using murine and cellular models, we have observed that the supramolecular structures of complex IV have different metabolic and physiological functions, ranging from organism viability, metabolic maturation necessary for proper tissue physiology, to plasticity and adaptation to different metabolic requirements.
Michaela Veliova, PhD

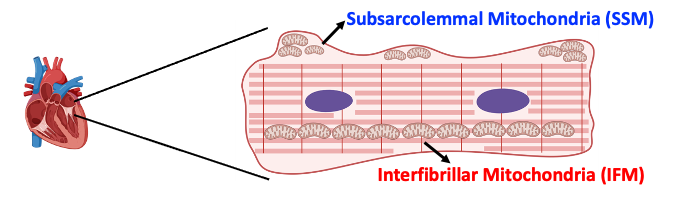
After completing my Ph.D. at the University of California, Los Angeles (UCLA), I joined the GENOXPHOS lab as a postdoctoral researcher to investigate the intracellular diversity of cardiomyocyte mitochondria. My research focuses on understanding the functional differences between interfibrillar mitochondria (IFM) and subsarcolemmal mitochondria (SSM). These distinct mitochondrial types—or mitotypes—are known to play critical roles in cardiomyocyte physiology, yet the mechanisms that enable their formation and maintenance within a single cell remain unclear.
Currently, I am exploring how cytoplasmic ribosomes may influence mitotype differentiation, aiming to uncover how differential protein expression is regulated across mitotypes and how the cellular microenvironment affects their proteomic profiles and functional characteristics. Through this work, I aim to reveal new insights into the processes that drive mitochondrial diversity and specialization within cardiomyocytes.
Rebeca Acín

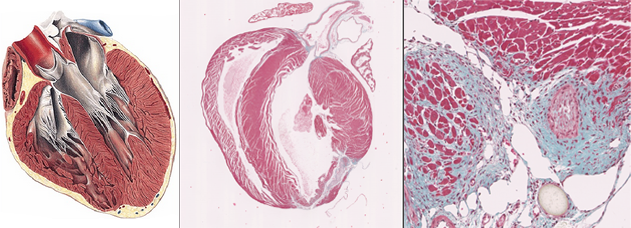
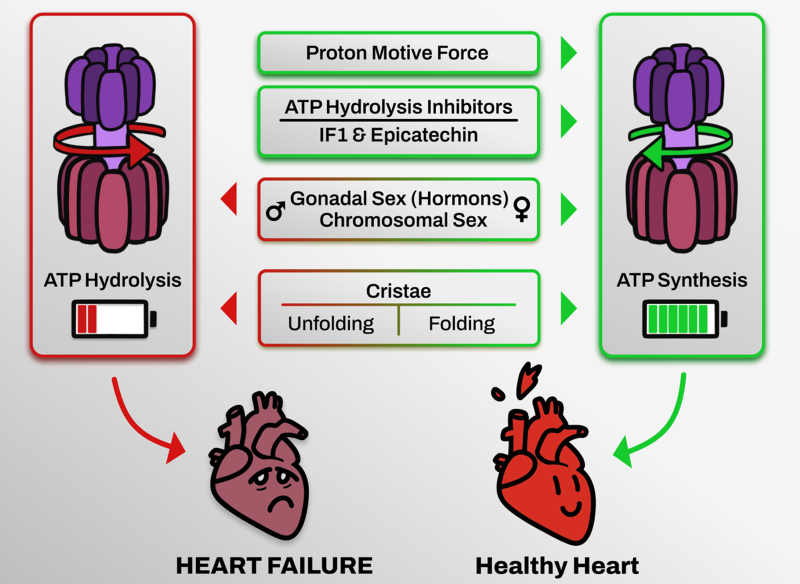
The research project I´m leading is focused in the study of the role of mitochondria in heart failure. In particular, the project is centered in understanding how ATP depletion (achieved by mitochondrial Complex V or ATPase working in reverse mode) is contribution to the adverse effects in cardiovascular diseases. Our group has recently demonstrated that Complex V ATP hydrolysis inhibition is beneficial in mitochondrial derived pathologies. Exploring the role of ATP hydrolysis in heart failure and being able to target it, will open new therapeutic pathways to treat cardiovascular diseases. This project will unravel the cellular and molecular differences that underlie distinct HF pathologies in males and females.
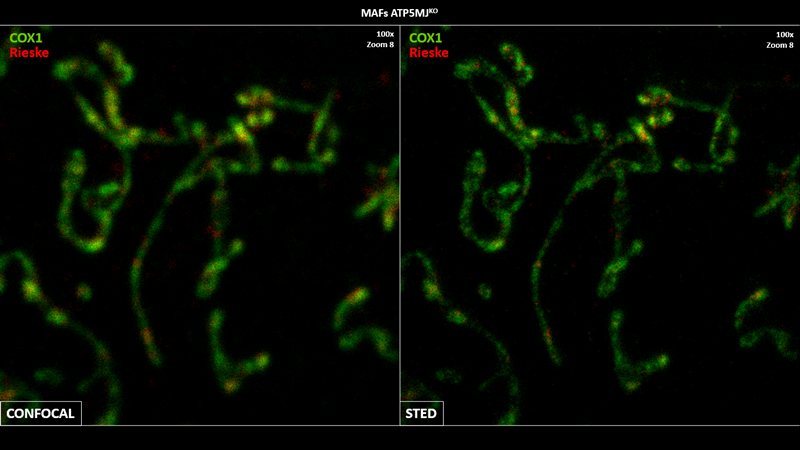
Sara Natalia Jaroszewicz

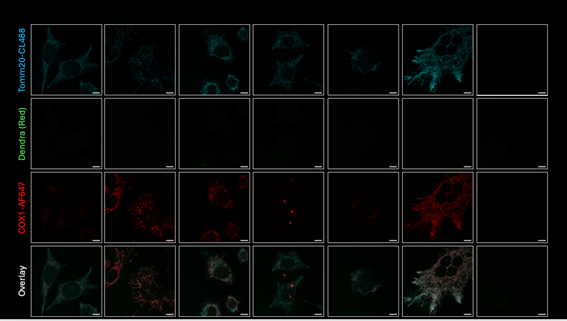
I graduated in Biochemistry from the Autonomous University of Madrid in 2022 and joined the group in the summer of that same year as a CICERONE internship student and later as a Master's student. My doctoral thesis consists of deciphering the in situ organization of the electron transport chain using super-resolution techniques.
Mitochondria are essential organelles in eukaryotic cellular metabolism. Oxidative phosphorylation occurs in them and this process is mediated by respiratory complexes (RCs), which can assemble into macromolecular structures known as supercomplexes (SCs). Although the existence of SCs has been demonstrated, there are still doubts about how these complexes are actually organized in the inner mitochondrial membrane and how their proportion could influence the development of heart diseases. To answer these questions, my project focuses on the optimization of STED, Expansion Microscopy (iU-ExM), proximity ligation assay (PLA) and cryoET super-resolution methods in order to visualize SCs in situ, determine their physiological position in subdomains of the mitochondrial cristae and compare them quantitatively in a murine model of heart failure.