Alberto Pascual: “Wanting to live forever is not the best thing for a species”
Spanish National Research Council (CSIC), Institute of Biomedicine of Seville (IBiS) and coordinator of the National Research Network on Hypoxia

Alberto Pascual holds a degree and doctorate in biology from the University of Seville. His thesis in biochemistry and molecular biology was about ribonuclease P, an RNA enzyme involved in the maturation of tRNAs. He took up a postdoctoral fellowship in France at the laboratory of Dr Thomas Preát. Pascual returned to the Spanish science system with a Ramón y Cajal contract (2003) at the Virgen del Rocío University Hospital (HUVR) in collaboration with Professor José López Barneo and obtained his position as CSIC senior scientist at IBiS in 2007. As principal investigator at IBiS (from 2007) he has pursued a new, independent line of research on Alzheimer’s disease (AD) and the hypoxia signalling pathway with a special focus on non-neural cells, in particular the endothelium and microglia. He has led regional, national and international projects with both public and private funding. He is currently secretary of SENC (the Spanish Society of Neuroscience), coordinator of the National Research Network on Hypoxia and a CSIC Scientific Researcher.
What do the brain, cardiovascular health and Alzheimer’s disease have in common?
Many cases of Alzheimer’s are related with genetic factors. They are very interesting to discover what the disease can be like and how it can progress. Genetic factors give us clues about how it develops but, of course, they are genetic and that cannot change. They are what we call unchangeable. However, there are many other factors associated with a higher risk of many types of dementia in general and Alzheimer’s specifically. For instance, there are some very surprising things that most people don’t know about, such as social isolation. Deafness in adults or schizophrenia multiply by almost 7 the chances of developing Alzheimer’s as you age. And we have no idea what could be happening.
It seems that, for the brain, social interaction is very demanding and when a person is using their brain to communicate and has to relate to others, it is kept active. It’s like a muscle, when you stop using it, it atrophies. The same thing happens to the brain.
As we get older and there are more problems with our body, it is likely that the brain does not receive the necessary attention due to cardiovascular problems and other types of abnormalities in the organism. And when you stop using it and don’t have enough energetic input, there are likely to be structural failures.
We are very interested in the idea of changeable risks because certain studies say that the incidence of Alzheimer’s could be reduced 35% just by lowering changeable risks.
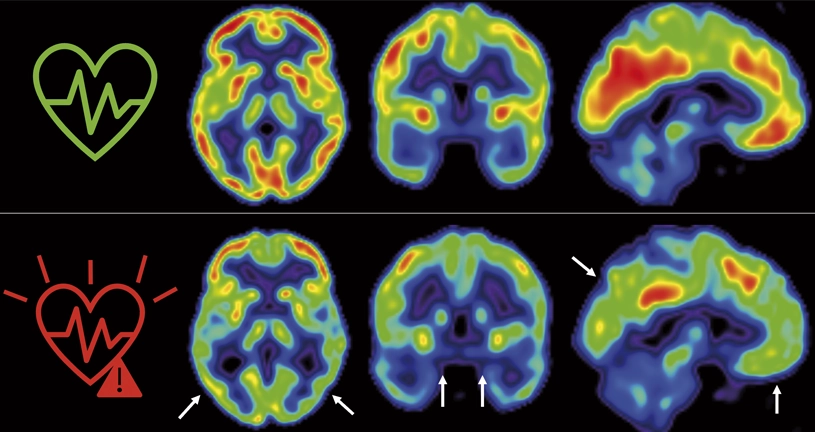
On the other hand, a large part of the risks associated with Alzheimer’s are cardiovascular factors; for instance, diabetes, atrial fibrillation, having had heart problems, atherosclerosis, even sleep apnoea or chronic obstructive pulmonary disease (COPD). All of these parameters increase the possibility of having dementia.
In the last 15-20 years many treatments have been developed to control atherosclerosis, high blood pressure or vascular function. In the wealthiest countries, most older people receive chronic treatment with this type of drug. If this is right, we should see a reduction in the incidence of Alzheimer’s in the years to come. Several recent review articles exist, and we observe that once we correct for age, which is the largest risk factor for the development of dementia, there is a reduction in the prevalence of Alzheimer’s in society. Which is to say that controlling cardiovascular risk seems to have a very interesting effect, not only on our daily lives, but also for the future. It is also protecting the integrity of the brain.
There are some very surprising things that most people don’t know about, such as social isolation. Deafness in adults or schizophrenia multiply by almost 7 the chances of developing Alzheimer’s as you age
And how can we begin to control the risk?
Through lifestyle. Curiously, in the population studies conducted in the USA, Sweden and Spain, we observe a reduction in the incidence and prevalence of Alzheimer’s, but not in Japanese one. Because Japan precisely has a diet and genetics that are not related with cardiovascular problems; that’s why we see no change.
But all of this is correlations. Our approach is different: we want to know if cardiovascular risks really affect the brain, how they change it and how they predispose to Alzheimer’s. The disease itself, how it interacts with the vasculature; i.e. the development of Alzheimer’s, which may be independent of cardiovascular risks: a lack of blood flow in the brain, a lack of nutrients and less oxygen also favour the situation, and how this affects brain function. These two lines are what we are working on.
In reality, conventional wisdom has it that when a person starts to forget things it’s because blood isn’t reaching their brain…
Yes, oxygen and nutrients. It’s very difficult to just reduce the amount of oxygen. There are situations in which a pulmonary obstruction, for instance, reduces the amount of oxygen. But really, if there is a reduction in the input of nutrients or oxygen, they go hand in hand. And we believe that this is interesting because the cell pathways that control them are completely different.
After much consideration, we are studying two main cell populations: one is blood vessels and how they change. We proposed a novel mechanism through which a disease can locally affect vessels and foster cardiovascular risks.
The next thing we did was to see how this affects the cells that defend us from disease, such as microglia, the immune system. And what we see is that precisely in these conditions, these cells are not able to respond to disease and over time they lose their capacity to protect. These studies were published in 2021.
Now, on the one hand, we are studying how activity of the immune system depends on mitochondria and is, therefore, dependent on a good supply of oxygen and nutrients. We have very interesting data, very similar to a study conducted here at CNIC by David Sancho with peripheral macrophages, which studied the function of mitochondria, and we are doing the same thing but with the macrophages found in the brain, microglia.
On the other hand, we are researching how to undo the vascular damage we find that is associated with disease. We have several approaches, one of which is pharmacological, that we don’t think is going to get very far just yet. What we see is that because of this lack of oxygen and nutrients, the vessels try to grow in the brain, which is a typical response to the loss of oxygen, and when they come across an amyloid protein, the angiogenesis is interrupted and the vessels that are being generated are lost, but the vessels they come from also die. Which is to say, the attempted recovery makes the disease worse. This process is called non-productive angiogenesis, and we are studying how to revert the situation genetically or pharmacologically.
It seems that for the brain, social interaction is very demanding and when a person is using their brain to communicate and has to relate to others, it’s kept active
Is there a drug that can do this?
We have seen that when angiogenesis begins it becomes unproductive and destroys the vessels that were there. The first hypothesis was to block angiogenesis so that the point of destruction of local vessels would not be reached. It would be a transitory rather than a long-term solution, We were lucky in that many antiangiogenic agents have been developed for cancer. We have managed to partially rescue the amyloid load and the behaviour of animals with memory defects.
However, it isn’t feasible to administer antiangiogenic drugs to an older person. The problem is that there aren’t many studies on angiogenesis in the brain.
In collaboration with Rui Benedito and Henar Cuervo, at CNIC we are currently testing genetically mending the molecular pathway that we know is destroyed. It’s about acting one step ahead. We have seen that the vessel doesn’t have a Notch pathway, a cellular signalling identity that goes a bit mad around the amyloid plaques, and under these conditions what we try to do is recover this Notch pathway. We do this genetically with viral vectors we have made in collaboration with Juan Bernal at CNIC’s viral production unit. With these virus we manage to recover memory behaviour and vascularization that had been lost around the plaques quite well.
Why could this be so important? Because amyloid accumulates in the bran, above all when there are problems clearing. Normally, when we sleep, we change the pressures in our body and sleep clears the amyloid from the brain. What is more associated with the accumulation of amyloid in humans is fragmented sleep; i.e. people who do not sleep continuously well or who get up several times during the night, their amyloid load increases.
To clear it we need the vascular system. If we have a vascular problem associated with amyloid deposits, that is a problem that aggregates. In the end, it’s a question of time and error, simply a series of situations that lead to a reduction of brain activity. We think that if we partially recover the vasculature, we could clear this amyloid and delay the onset of a disease.
As a joke, I usually say that the biggest contribution to the onset of Alzheimer’s was the invention of antibiotics. We have prolonged life, and we have other problems that we weren’t prepared for.
Our immune system and defences are in a state of natural selection during the time we are active and fertile, maybe up to the age of 50. From then on, life normally doesn’t continue much longer.
Our immune system and defences are in a state of natural selection during the time we are active and fertile, maybe up to the age of 50. From then on life normally doesn’t continue much longer
In some ways, the machine was not prepared to live so long.
It’s not that we have a sell-by date. It’s that the selection of things needed to live for 40 or 50 years has been made; what comes after was irrelevant.
But some say our lifespans will get increasingly longer…
Not just that, you have to keep your body healthy over the age of 80. Evolution is the selection of what works, but of course, what works for the times you live in. For instance, our previous director, José López Barneo, often says that people have already been born that are going to live 130 or 140 years.
There is debate in the field between people who say there is a maximum number of years we can live and that, however much we optimize our life with healthy habits, medicine, etc, the species has a limit. Longevity really depends on the size of the species. Small species have a very fast metabolism and live short spans, whereas larger species who have a slower metabolism live many more years. So maybe what we have to do is live slower, but how can we do that? As the poet said, “I want to die living,” it’s not a case of anything goes. What I mean is, everyone wants to live longer, but in good conditions and with quality of life.
And then there is the ethical debate. How far can we prolong life and in what conditions? We live in an overpopulated world. Wanting to live forever is not the best thing for a species. I don’t think we are the best thing for the planet either.