Excellence in communication science
The main scientific journals científicas publish research from the CNIC laboratories
EMBO Molecular Medicine
Accumulation of the protein versican is the cause of aortic aneurysm in Marfan syndrome
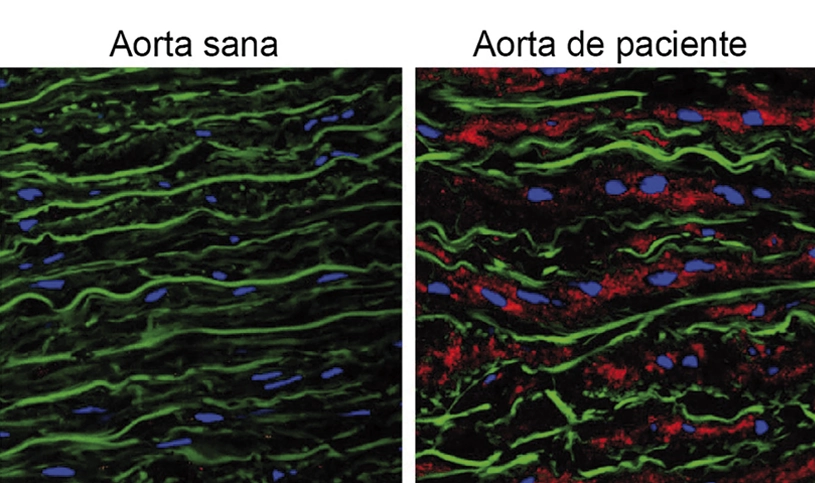
A team of Spanish scientists has identified the cause of aortic aneurysm in patients diagnosed with Marfan syndrome, a genetic disorder currently lacking treatment options. The study, published in the journal EMBO Molecular Medicine, was conducted by researchers at the CNIC, in collaboration with partners at the Centro de Biología Molecular Severo Ochoa (CBMSO, CSIC).
The study, led by CBMSO scientists Juan Miguel Redondo and Miguel R. Campanero, reports an accumulation of a proteoglycan called versican—a large protein present in the extracellular matrix between cells—in the aortas of patients with Marfan syndrome and of mice genetically engineered to develop the disease, known as Marfan mice.
The new discovery identifies versican accumulation as a cause of the aortic aneurysms suffered by Marfan syndrome patients. The study also identifies the signaling pathway mediated by protein kinase B, known as AKT, as a possible target for treating aortic disease in Marfan syndrome.
These results represent an important advance in the understanding and of the aortic disease associated with Marfan syndrome. An aortic aneurysm is an enlargement of the aorta caused by a weakening of its wall. Although initially asymptomatic, aortic aneurysm can lead to serious complications such as dissection or rupture of the aortic wall, which can be fatal. “Marfan syndrome is a genetic disorder affecting connective tissue, and thoracic aneurysm and aortic dissection [TAAD] is the main cause of death in these patients,” explained Redondo. The study also shows that decreasing versican expression by gene silencing reduces the expression of Nos2 and completely reverses aortic disease in Marfan mice.
The identification of AKT as a possible therapeutic target for the aortic pathology in Marfan syndrome is a very promising finding that could help in the development of new treatments for this disease.
The study was supported by grants from the Marfan Foundation, the MERCK Foundation-Spanish Foundation for Rare Diseases 2022, and the V-Ayudas “Muévete por los que no pueden 2021” program, as well as professional training contracts from the Spanish Ministry of Science and Innovation.
Science Advances
First therapeutic target for preserving heart function in patients with pulmonary hypertension

A team led by Dr. Guadalupe Sabio at the CNIC has discovered a possible therapeutic target for pulmonary hypertension. The study, published in the journal ‘Science Advances’, identifies the first therapeutic target that can be modulated to preserve cardiac function in pulmonary hypertension, providing hope in the fight against this rare but fatal disease for which there is currently no cure.
Pulmonary hypertension is a condition of elevated blood pressure in the arteries that carry deoxygenated blood to the lungs. This increased pulmonary blood pressure puts the heart under continuous strain as it has to work harder to pump blood to the lungs.
Pulmonary hypertension affects between 15 and 50 people per million of the world population. In Spain, the estimated prevalence is 1.6 cases per 100,000 inhabitants, and the estimated incidence (new cases diagnosed per year) is 0.3 per 100,000 inhabitants.
Currently available treatments target the lungs, aiming to lower blood pressure. However, these strategies do not improve cardiac function, making heart failure the main cause of death in these patients.
The CNIC researchers found that patients with chronic obstructive pulmonary disease (COPD) have elevated levels of a mitochondrial protein called MCJ.
The study results demonstrate that modulating the levels of MCJ in the heart can preserve cardiac function despite the presence of lung injury.
The study was supported by grants from Ministerio de Ciencia e Innovación (RED2022-134397-T, MINECO-PID2019-104399RB-I00, PGC2018-097019-B-I00), IMPACT-2021 PROJECT (PMP21/00057), Fundación Jesús Serra, EFSD/Lilly European Diabetes Research Programme, Fundación BBVA, Comunidad de Madrid and AECC.Circulation Research
APOE genetic variants linked to Alzheimer disease are also associated with the development of subclinical atherosclerosis

IScientists at the CNIC have found that one of the most potent genetic risk factors for Alzheimer disease, apolipoprotein E4 (APOE4), is also associated with an increased risk of developing subclinical atherosclerosis in middle age. The study also demonstrates protection against subclinical atherosclerosis in people carrying the variant APOE2, which protects against Alzheimer disease.
The APOE gene encodes apolipoprotein E, which, among other important functions, contributes to the transport of lipids in the blood. There are three main APOE alleles, which give rise to three apolipoprotein isoforms: APOE2, APOE3, and APOE4. “Inheriting one or other of these alleles confers a different risk of developing distinct diseases, among them cardiovascular disease and Alzheimer disease,” explained Dr. Cortés Canteli, a neuroscientist at the CNIC and a Miguel Servet fellow at the Fundación Jiménez Díaz University Hospital Health Research Institute.
In the new study, the CNIC team demonstrates that APOE4 carriers among the middle-aged participants in the PESA-CNIC-Santander study (aged 40–54 years) have elevated levels of circulating LDL cholesterol (‘bad’ cholesterol), which increases their risk of developing subclinical atherosclerosis. This finding provides a window of opportunity for implementing early intervention strategies.
The study also shows that carriers of the APOE2 variant have comparatively less subclinical atherosclerosis in the carotid, femoral, and coronary arteries. This protection is due to these individuals having normal levels of triglycerides or, in the case of women and individuals in the youngest age category (40–44 years), comparatively low levels of circulating LDL cholesterol. “These findings underline, once more, the importance of a healthy lifestyle,” stressed Dr. Fuster, who combines his role at the CNIC with those of President of the Cardiovascular Institute and Physician-in-Chief at Mount Sinai Medical Center in New York. The present study received funding from the European Regional Development Fund (EDRF–A way to build Europe) and the European Social Fund (ESF–Investing in your future).
The PESA study is cofinanced equally by the CNIC and Santander Bank. The study also receives financial support from the ISCIII (PI15/02019, PI17/00590 & PI20/00819) and for the present study in particular has received funding from the BrightFocus Foundation. The present study involved the participation of investigators from the Spanish research networks for cardiovascular biomedicine (CiberCV) and rare diseases (CiberRER).
The research was funded by the European Research Council (No 866240, JFB), the Ministry of Science and Innovation (PID2019-108568RB-I00, JFB), and the Novo Nordisk Foundation in Denmark (NNF17OC0030688, JFB).
Nature Cardiovascular Research
New approach to the design of therapies that enhance the effect of cholesterol-lowering drugs

A research team from the CNIC in Madrid, in collaboration with Aarhus University in Denmark, has uncovered a crucial mechanism that leads to the regression, or shrinkage, of atherosclerotic plaques. This discovery, published in Nature Cardiovascular Research, highlights smooth muscle cell-derived cells in the arterial wall as a promising target for future therapies aimed at reducing plaque growth in advanced atherosclerosis.
The mechanism identified involves inflammatory signaling within a subset of smooth muscle cells responsible for the development of atherosclerotic plaques. Jacob F. Bentzon, leader of the research teams at both institutions, pointed out that this discovery could lead to targeted therapies that enhance the effectiveness of cholesterol-lowering drugs and improve plaque regression in patients with advanced cardiovascular disease.
Smooth muscle cells, which are a core part of the arterial wall, play a key role in the progression of cardiovascular diseases such as heart attack and stroke. Their proliferation and transformation during atherosclerosis contribute significantly to these disorders.
High blood cholesterol is the main cause of atherosclerosis, and lifestyle changes or medications like statins are effective ways to prevent the condition. In patients with advanced atherosclerosis, reducing cholesterol levels lowers the risk of irreversible plaque formation, but the precise mechanisms behind this reduction have not been fully understood.
The study demonstrates that, when cholesterol levels are lowered in mice with advanced atherosclerosis, certain harmful smooth muscle-derived cells in the plaques diminish, while beneficial cells that stabilize the plaques remain. This finding, highlighted by Laura Carramolino, the first author, points to the potential for more effective therapeutic strategies targeting these cells to prevent severe outcomes like heart attacks or strokes.
The research was funded by the European Research Council (No 866240, JFB), the Ministry of Science and Innovation (PID2019-108568RB-I00, JFB), and the Novo Nordisk Foundation in Denmark (NNF17OC0030688, JFB).
Circulation Research
New mechanism discovered for the life-threatening arrhythmias in Andersen-Tawil syndrome

A team at the CNIC, led by Dr. José Jalife, has made a significant breakthrough in understanding the genetic basis of cardiac arrhythmias, particularly related to the rare Andersen-Tawil syndrome (ATS). Their research, published in Circulation Research, reveals how a specific genetic mutation (C122Y) in the Kir2.1 potassium channel not only disrupts the function of Kir2.1 itself but also impairs the main cardiac sodium channel, NaV1.5. This discovery establishes a direct link between the mutation and the life-threatening arrhythmias characteristic of ATS1. The study shows that the C122Y mutation in the Kir2.1 channel has a dual impact: it causes a reorganization of Kir2.1 that weakens its binding to the phospholipid PIP2, an essential component for cellular signaling in the membrane, while also disrupting the stability and expression of the NaV1.5 protein. Both Kir2.1 and NaV1.5 are critical for maintaining proper heart rhythm, and disturbances in either channel can trigger severe arrhythmias. Although cardiac arrhythmia is a common condition, affecting about 1 in 3 people at some point in their life, ATS is extremely rare, with fewer than 1 in a million affected. ATS1 is caused by mutations in the KCNJ2 gene, which encodes the Kir2.1 channel. Patients with ATS1 experience a unique combination of symptoms, including periodic paralysis, arrhythmias, and distinctive facial features. The disorder is inherited in an autosomal dominant manner.
Using a mouse model that replicates the electrical abnormalities seen in ATS1 patients, the researchers found that the mutation not only impacts the Kir2.1 channel but also interferes with the function of the NaV1.5 channel, essential for cardiac excitability. This disruption of two key ion channels helps explain the severe arrhythmias observed in ATS1 patients.
This discovery represents a critical step forward in improving clinical approaches to arrhythmias, offering hope for more effective, individualized treatments that could benefit millions of people worldwide. The study was funded by the National Heart, Lung, and Blood Institute of the NIH (USA); the la Caixa Foundation; the La Marató de TV3 Foundation; the Spanish cardiovascular research network (CIBERCV); the Horizon 2020 Programme of the European Union; and Program S2022/BMD7229. The imaging studies were performed at the TRIMA@CNIC node of the Distributed Biomedical Imaging Network (ICTS ReDIB).
JACC: CardioOncology
CNIC scientists identify therapeutic targets for the prevention of heart injury linked to cancer treatment

Scientists at the CNIC have identified the mechanisms through which anthracyclines, a widely used class of anticancer drugs, damage the hearts of patients receiving this treatment. The study, published in the journal JACC: CardioOncology, also identifies possible treatments for this complication, which affects an estimated one third of cancer survivors. Over 4 million Europeans are diagnosed with cancer annually, and while survival rates have improved, treatments like anthracyclines, used in about 3 million patients, pose significant risks. These drugs are cardiotoxic, with one-third of patients experiencing heart damage. In more than 5% of survivors, this leads to chronic heart failure, severely impacting their quality of life. Despite this, no specific treatments to protect the heart have been developed, due to limited understanding of how anthracyclines cause cardiac injury.
Researchers at the CNIC, led by Dr. Borja Ibáñez, have identified how anthracycline chemotherapy damages the heart by disrupting cardiac metabolism, with a focus on mitochondrial dysfunction. Their detailed analysis in an experimental animal model revealed that anthracyclines cause early and irreversible changes in the heart’s energy supply from fatty acids and glucose, leading to impaired energy production in the mitochondria. These metabolic alterations occur early in treatment, well before any visible loss of heart contractile function, and result in the atrophy of heart cells as an early sign of irreversible damage. This discovery is crucial because the metabolic changes can be detected long before traditional methods reveal heart damage. By understanding the molecular alterations behind this process, the researchers identified potential points where early intervention could prevent damage. One promising approach under investigation involves a protein-enriched diet to prevent muscle atrophy, including cardiac muscle, caused by anthracycline treatment.
The CNIC is committed to finding solutions to unresolved clinical needs and, within its Programa de Homeostasis Miocárdica y Daño Cardiaco, has set up a research line dedicated to chemotherapy-associated cardiotoxicity, with a particular focus on anthracyclines. The goal is to develop treatments that maintain the efficacy of the anticancer treatment while minimizing negative impacts on cardiovascular health. Dr. Ibañez’s research group also coordinates projects financed by the European Commission (ERC-Consolidator “MATRIX”, y Horizon2020-HEALTH “RESILIENCE”), both aimed at reducing the prevalence of heart failure among cancer survivors. Imbued with a translational and multidisciplinary vision, these projects are being conducted through partnerships between the CNIC, Fundación Jiménez Díaz University Hospital, and the CIBERCV.
The current study received support from the European Commission (ERC-CoG 819775 and H2020-HEALTH 945118), the Spanish Ministry of Science, Innovation, and Universities (PID2022-140176OB-I00), and the Community of Madrid regional government through the Madrid Network for Nanomedicine in Molecular Imaging (P2022/BMD-7403 RENIM-CM).
JACC
A new Spanish study provides the first stratification of the risk of developing dilated cardiomyopathy among symptom-free genetic carriers
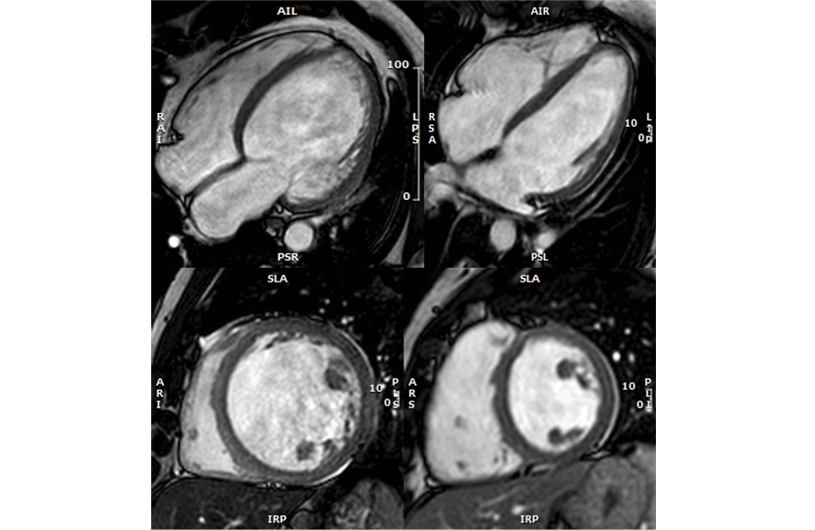
Dilated cardiomyopathy is the leading cause of heart failure in young people and a major reason for heart transplants. This condition causes the heart to enlarge and lose its ability to effectively pump blood, putting patients at high risk for arrhythmias and sudden death. In 30%–40% of cases, the disease is linked to genetic mutations, and identifying these mutations allows doctors to screen family members for the altered gene.
Family members who carry the mutation are at risk of developing the disease, making regular check-ups crucial for early detection and treatment. However, it remains unclear whether all carriers will develop the disease, at what age this is more likely, and which factors could predict disease onset in the short term, highlighting the need for more precise monitoring strategies. The new study, led by Dr. Pablo García-Pavía, a researcher at the CNIC, a group leader in the Spanish cardiovascular research network (CIBERCV), and a cardiologist at Hospital Puerta de Hierro, provides the first stratification of the risk of developing dilated cardiomyopathy among symptom-free genetic carriers of the disease.
A total of 25 Spanish hospitals participated in the study, which is published in the Journal of the American College of Cardiology. Data were collected from more than 779 genetic carriers, from 300 families, who had shown no signs of the disease before entering the study. The study also showed that the appearance of the disease was dependent on the specific type of genetic mutation present.The study was supported by the Sociedad Española de Cardiología (a Hereditary Cardiac Disease grant awarded in 2022) and the Instituto de Salud Carlos III through projects PI18/0004 and PI20/0320 (cofounded by the European Regional Development Fund/European Social Fund “A way to build Europe”/“Investing in your Future”).
PNAS
CNIC scientists identify the key cell type for strategies to prevent atherosclerosis in progeria syndrome

Hutchinson-Gilford progeria syndrome (HGPS) is an extremely rare genetic disease that causes accelerated aging, severe atherosclerosis, and premature death at an average age of 15 years. Despite the absence of typical cardiovascular risk factors, premature atherosclerosis is the leading cause of death. HGPS is caused by a mutation in the LMNA gene that produces progerin, a harmful version of the lamin A protein.
Recent research has shown that gene editing can correct this mutation, eliminating progerin and restoring lamin A, which improves symptoms and extends life in animal models. A study published in PNAS investigated the effectiveness of removing progerin in endothelial cells and vascular smooth muscle cells, two key cell types in atherosclerosis. The results indicated that removing progerin in endothelial cells did not provide benefits, while its removal in vascular smooth muscle cells significantly reduced atherosclerosis and other complications. These findings suggest that targeting gene therapy to vascular smooth muscle cells could be sufficient to treat HGPS-related atherosclerosis, potentially using lower doses of gene-editing reagents.
The study was funded by the Ministerio de Ciencia, Innovación y Universidades (MICIU)/Agencia Estatal de Investigación (AEI)/10.13039/501100011033 and ERDF/EU (grants PID2022-141211OB-I00 and PID2022-137111OA-I00); the Comunidad Autónoma de Madrid (grants 2017-T1/BMD-5247 and 2021-5A/BMD-20944) cofinanced with European structural and investment funds; RYC2021-033805-I (MICIU/AEI/10.13039/501100011033 and European Union NextGenerationEU/PRTR); the Ministerio de Educación, Cultura y Deporte; Fundación “la Caixa”; and the Wellcome Trust. The CNIC receives institution-level support from the Instituto de Salud Carlos III (ISCIII), the MICIU, and the Pro-CNIC Foundation, and is a Severo Ochoa Center of Excellence (award CEX2020-001041-S funded by MICIU/AEI/10.13039/501100011033).
Development Cell
A CNIC study reveals the key role of mitochondrial proteins in cardiac regeneration

A study by CNIC and the University of Bern has revealed new insights into the role of mitochondria in heart regeneration. Published in Development Cell, the research, led by Dr. José Antonio Enríquez and Dr. Nadia Mercader, identifies the cox7a protein family as crucial for the assembly of complex IV (CIV) in the mitochondrial respiratory chain, which is essential for cellular energy production.
The research focused on three members of this family: Cox7a1, Cox7a2, and Cox7a2l (SCAF1). The scientists found that Cox7a1 is key to forming CIV dimers. Using a zebrafish model, they observed that the absence of Cox7a1 negatively affected body weight and swimming ability, but also enhanced the heart’s regenerative response after cardiac injury. The study showed that the loss of Cox7a1 in the heart improves its recovery capacity after damage, suggesting that these proteins influence cardiac regeneration. Additionally, significant metabolic changes were identified in the muscles of fish lacking Cox7a1, which could have implications for the treatment of heart and metabolic diseases. This finding represents a major advance in understanding heart regeneration and suggests that mitochondrial assembly proteins may play a key role in controlling cellular metabolism. The study was supported by the European Union Horizon 2020 programme (grants 874764 and 819717), the Human Frontier Science Program (grant RGP0016/2018), and the Swiss National Science Foundation (grant 320030E-164245).
Nucleic Acids Research
A CNIC team creates an innovative tool to study the function of genes in a safer and more effective way

A team from the CNIC, led by Rui Benedito, has developed a new genetic tool called iSuRe-HadCre, which improves the precision and reliability of genetic alterations in tissues or individual cells. Published in Nucleic Acids Research, this technology overcomes the limitations of the Cre-Lox system, which has been traditionally used for gene function analysis.
The Cre-Lox system has been essential in biomedical research due to its ability to manipulate gene expression in a spatial and temporal manner. However, it presents issues such as variability in recombination efficiency and the need for costly controls to ensure proper experimental execution. The new iSuRe-HadCre tool uses an inducible double-recombinase genetic cascade that ensures cells with a fluorescent marker have experienced high Cre activity but no longer maintain it. This allows for precise genetic alterations and eliminates the toxicity and sporadic recombinations associated with constant Cre expression. Additionally, iSuRe-HadCre is more sensitive to induction by CreERT2 and tamoxifen.
Rui Benedito emphasizes that iSuRe-HadCre is a key tool for advanced genetic studies, including high-resolution microscopy, functional analyses, and genetic epistasis studies, allowing for simultaneous genetic modifications with high efficiency. The study was funded by the Ministerio de Ciencia e Innovación, “la Caixa” Foundation, the European Research Council, the Leducq Foundation, the Knut and Alice Wallenberg Foundation, and the Göran Gustafsson Foundation.













