Excellence in Communication Science
Leading journals publish CNIC science
JAMA CARDIOLOGY
An international team identifies the mutations that cause the most frequent congenital heart defects

Bicuspid aortic valve is the most common congenital defect in humans, affecting between 1% and 2% of the population. Instead of the usual three symmetric leaflets, affected individuals have two asymmetric valve leaflets. This defect is a frequent cause of aortic stenosis and endocarditis and is associated with early calcification of the aortic valve. Currently the only effective treatment is valve replacement surgery.
But this situation could be changed by the results of a new study published by an international team co-led by CNIC group leader Dr. José Luis de la Pompa.
This innovative multicenter study, published in JAMA Cardiology, reveals that biscuspid aortic valve is cause by mutations in the MINDBOMB1 gene (MIB1), some of them described for the first time in the new study and others previously reported by the same group in an earlier article in Nature Medicine.
The study combined genome sequencing, the sequencing of candidate genes in a familial cohort, analysis of the association of rare variants in additional cohorts, and further analysis of the association of common variants in a third, large cohort, explained Idit Tessler of Sheba Hospital, a co-leader on the study. The analysis of mutations in patients from different populations strengthens the validity of the study.
To analyze the specific mechanisms through which MIB1 ensures correct heart development, Dr. Rebeca Piñeiro-Sabarís from the team at CNIC, led by Dr. José Luis de la Pompa and co-first author of the study, used CRISPR-Cas9 gene editing to introduce the identified mutations into the sensitized genome of mice carrying one mutant allele for the NOTCH receptor. Both mutations (double heterozygosis) were required for the mice to develop bicuspid aortic valve at a high rate, contrasting with the development of the heart defect in human patients with a single mutation in one MIB1 allele (single heterozygotes). The mice carrying both mutations also had defects in the interventricular septum. This study is part of Dr. Rebeca Piñeiro-Sabarís’ doctoral thesis.
The researchers conclude that the identified association between MIB1 and bicuspid aortic valve highlights the important role of the NOTCH signaling pathway in this congenital defect and the potential of NOTCH pathway components as targets for the design of new diagnostic and therapeutic strategies.
The study was funded by the Ministerio de Ciencia e Innovación (MICIN).
JAMA CARDIOLOGY
Promoting health in high-school students: a randomized group intervention trial
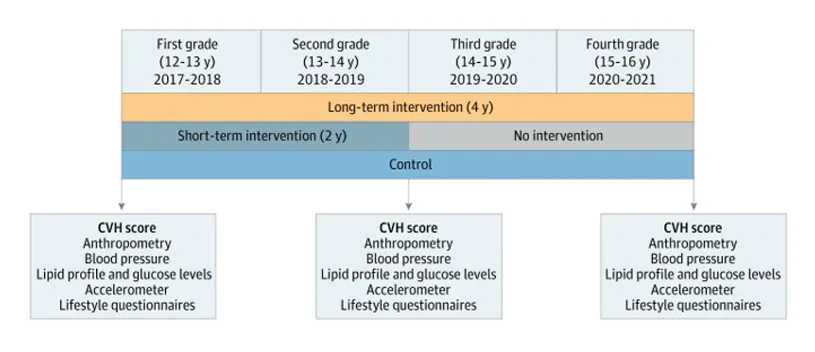
JAMA Cardiology has published the final results of a health-promotion intervention designed and carried out by the SHE Foundation, the CNIC, and the University of Barcelona involving the participation of 1326 adolescents from 24 state secondary schools in Madrid and Barcelona.
The results show a beneficial impact that depended to a large extent on the intensity of the educational intervention. Unfortunately, the beneficial impact was not sustained over time.
The SI! (Salud Integral) Program for the promotion of cardiovascular health in the school environment is an educational intervention designed by the SHE Foundation with support from Fundación “la Caixa” and is directed by CNIC Director Dr. Valentín Fuster. The program covers the preschool, primary, and secondary educational stages and includes girls and boys between the ages of 3 and 16 years. Since 2009, the SI! Program has been assessed in randomized intervention trials in 3 countries with differing socioeconomic contexts: Colombia, Spain, and the USA. These studies have included a total of approximately 7000 boys and girls.
The program covers 4 fundamental and interrelated components: diet, physical activity, knowledge of the body and heart, and emotions management. Content and strategy are adapted to each age group and include the whole educational community of students, teachers, and families and also consider the school environment.
The new study reports the results of a school-based health-promotion intervention in more than 1000 adolescents between the ages of 12 and 16 years. The main goal was to assess the impact on lifestyle and health parameters of 2 versions of the SI! Program secondary school intervention that differed in their duration and intensity.
The 24 participating high schools were randomized to intervention group (SI! Program intervention during the first 2 years of high school or throughout the 4-year secondary education period) or to a control group that continued with the schools’ usual study plans.
To assess the cardiovascular health of the participating adolescents before, during, and after the intervention, the study used the cardiovascular health metric recommended by the American Heart Association. This metric covers lifestyle habits and health parameters such as body weight, diet, physical activity, smoking, blood pressure, and blood cholesterol and glucose concentrations.
The longer version of the SI! Program for Secondary Schools intervention showed a significant improvement in the cardiovascular health of adolescents when assessed at the midway point. In contrast, the more intensive 2-year intervention produced no significant effects.
The second phase of the 4-year intervention was affected by the Covid-19 pandemic, and the results of the final evaluation therefore need to be interpreted with caution.
Although health promotion initiatives have become increasingly routine in the school environment in recent years, very few have addressed overall cardiovascular health, and it is therefore important to continue to improve school-based interventions in order to define the most effective strategies.
The study was supported by Fundación SHE-Fundación la Caixa, the Instituto de Salud Carlos III (ISCIIII), and Fundació La Marató de TV3.
NATURE COMMUNICATIONS
A new technique offers improved diagnostic precision and a route to personalized therapy for a common arrhythmia that affects more than 10 million people in Europe

A multidisciplinary study led by scientists at the CNIC presents a new method for assessing the structural and electrophysiological changes, called atrial remodeling, produced in the heart of patients with atrial fibrillation, one of the most frequent forms of cardiac arrhythmia. The new diagnostic method is based on the simultaneous assessment of electrical and mechanical (contractile) activity in the heart atria during atrial fibrillation. The study is published in Nature Communications.
Study leader David Filgueiras explained that, until now, “this was an unmet challenge,” because, on the one hand, “the available technology did not allow the integration of both types of information to provide a more complete evaluation,” and on the other hand, “during atrial fibrillation, the contractile movements of the atria are of low intensity, and measuring them is technically challenging.”
Atrial fibrillation is an irregular and often very fast heartbeat that can cause the formation of blood clots in the heart, increasing the risk of stroke, heart failure, and related complications. Atrial fibrillation condition affects an estimated 10 million people in Europe, and around 700,000 in Spain.
According to Dr. Filgueiras, “the importance of this new diagnostic method is its ability to provide a personalized assessment of an individual patient’s degree of atrial remodeling, independently of the clinical classification based on temporal criteria.”
The three first authors on the study are Daniel Enríquez Vázquez, of the Complejo Hospitalario Universitario in A Coruña and a member of the Spanish cardiovascular research network (CIBERCV), and CNIC scientists Jorge G. Quintanilla and Alba García Escolano. Dr. Enríquez Vázquez highlighted that “on a clinical level, the results show that electromechanical dissociation in patients with atrial fibrillation is a solid indicator of disease progression and of the need to take urgent steps to return these patients to normal rhythm in an efficient and stable manner.”
The team led by Dr. Filgueiras worked in partnership with colleagues at the Hospital Clínico San Carlos, the Hospital Universitario Central de Asturias, the Hospital de la Santa Creu i Sant Pau, the Complejo Hospitalario Universitario de A Coruña, the Universidad Complutense de Madrid, the Universidad Politécnica de Madrid, the Universidad Autónoma de Barcelona, the University of Connecticut, and the CIBERCV. Over the past 10 years, this team of national and international experts worked together to integrate electrical and mechanical cardiac data to enable a personalized characterization of the status of the pathological changes associated with the progression of atrial fibrillation.
In the first phase, engineers and physicists devised the most appropriate strategy for integrating the electrical and mechanical data. The solution they found was to measure mechanical activity by Doppler imaging—a noninvasive method that provides information on atrial tissue movements—and electrical activity by surface electrocardiography.
Both approaches are easy to implement in the clinic because they are noninvasive and can be conducted during a transthoracic ultrasound examination, a routine study of the shape and functioning of the heart and some of its internal structures.
The second phase involved experts in biology, biotechnology, biochemistry, and biomedical engineering working together with the CNIC Proteomics Unit and clinical cardiologists. Experimental studies conducted in this phase correlated the information obtained with the new approach with underlying pathological changes in atrial tissue. This information was used to develop new advanced mapping techniques and computer simulations to reveal the mechanisms underlying electrical and mechanical remodeling during the progression of atrial fibrillation.
The final phase was a multicenter prospective study of 83 patients at an early stage in the development of atrial fibrillation, to determine the prognostic value of simultaneous the electrical and mechanical assessment of the atria in patients with this type of arrhythmia.
The experimental and clinical findings revealed an imbalance between electrical and mechanical (contractile) activation in the atria at early stages of the disease. This causes the two parameters to become dissociated, so that the contractile activation cannot keep up with the electrical activation, a phenomenon the investigators call atrial electromechanical dissociation. The pace of this dissociation is specific to each individual patient, although in the absence of restoration of a normal rhythm it is usually observed within the first 2-3 months after an uninterrupted atrial fibrillation episode.
A key advantage of the new approach is that atrial electromechanical dissociation is identified before the appearance of overt clinical signs of structural atrial remodeling. “The use of this new diagnostic approach allows early characterization of the underlying remodeling in patients with atrial fibrillation,” said Dr. Filgueiras. “The study shows that it is possible to integrate electrical and mechanical data from the atria of patients with atrial fibrillation to obtain personalized prognostic information about the clinical progression of the disease.”
The study was supported by funding from the European Union H2020 Programme (Grant Agreement#965286), the Ministry of Science and Innovation (PID2019-109329RB-I00 and PGC2018-097019-B-I00), Instituto de Salud Carlos III (Fondo Europeo de Investigación Sanitaria PRB3 (PT17/0019/0003- ISCIII-SGEFI / ERDF, ProteoRed), the Interhospital Fund for Cardiovascular Research, CIBERCV, Fundación Salud 2000, and Fundación La Caixa (Project code HR17-00247).
THE LANCET HEALTHY LONGEVITY
Early action to control cardiovascular risk factors preserves brain metabolism
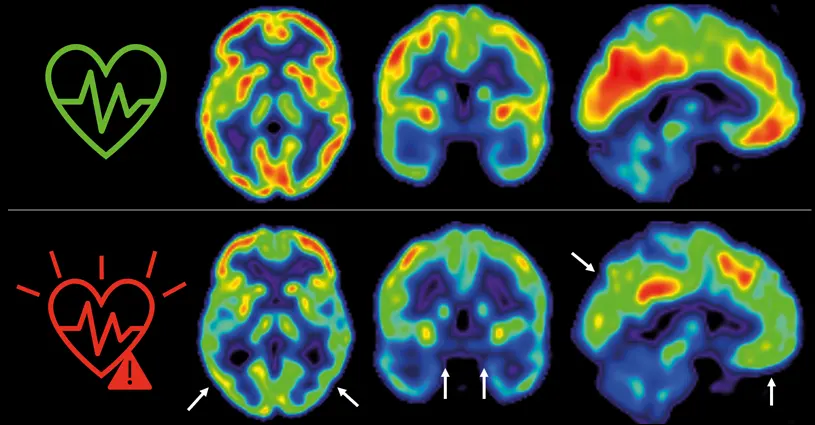
Cardiovascular disease and dementia frequently occur together in elderly people. Nevertheless, few longitudinal studies have examined how atherosclerosis and its associated risk factors affect brain health from middle age. Now, a new study by scientists at CNIC provides new data on this relationship; the results confirm the importance of controlling traditional cardiovascular risk factors, such as hypertension, cholesterol, diabetes, smoking, and a sedentary lifestyle, not only to preserve cardiovascular health, but also to prevent Alzheimer’s disease and other dementias.
Published in The Lancet Healthy Longevity, the CNIC study shows that atherosclerosis—the accumulation of fatty deposits in the arteries—and its associated risk factors, in addition to being the main cause of cardiovascular disease, are also implicated in the cerebral alterations typically found in Alzheimer’s disease, the most frequent cause of dementia.
According to CNIC General Director Dr. Valentín Fuster, an author on the study, the new findings are important because they open up the possibility of treating a modifiable disorder (cardiovascular disease) to prevent the development of a disease for which there is currently no curative treatment (dementia). “The sooner we act to control cardiovascular risk factors, the better it is for our brain health,” said Dr. Fuster.
Cardiovascular risk factors are important for preventing a heart attack,” continued Dr. Fuster. “Nevertheless, the additional information linking the same risk factors to a decline in brain health could further increase awareness of the need to acquire healthy habits from the earliest life stages.”
In 2021, CNIC scientists discovered that the presence of cardiovascular risk factors and subclinical (presymptomatic) atherosclerosis in the carotid arteries (the arteries that supply the brain) was associated with lower glucose metabolism in the brains of apparently healthy 50-year-old participants in the PESA-CNIC-Santander study (Cortés-Canteli & Gispert et al. JACC. 2021). Glucose metabolism in the brain is considered an indicator of brain health.
The PESA-CNIC-Santander, study, directed by Dr. Fuster, is a prospective study that includes more than 4000 asymptomatic middle-aged participants who have been exhaustively assessed for the presence and progression of subclinical atherosclerosis since 2010.
Dr. Fuster’s team, led by Drs. Marta Cortés Canteli and Juan Domingo Gispert, have continued to monitor the cerebral health of these participants over 5 years. Their research shows that individuals who maintained a high cardiovascular risk throughout this period had a more pronounced reduction in cerebral glucose metabolism, detected using imaging techniques such as positron emission tomography (PET).
“In participants with a sustained high cardiovascular risk, the decline in cerebral metabolism was three times greater than in participants who maintained a low cardiovascular risk,” commented Catarina Tristão-Pereira, first author on the study and INPhINIT fellow.
Glucose is the main energy source for neurons and other brain cells. “If there is a sustained decline in cerebral glucose consumption over several years, this may limit the brain ability to withstand neurodegenerative or cerebrovascular diseases in the future,” explained Dr. Gispert, an expert in neuroimaging at the CNIC and Barcelonaßeta Research Center.
Through a collaboration with Drs Henrik Zetterberg and Kaj Blennow, world experts in the identification of new blood biomarkers at the University of Gothenburg in Sweden, the CNIC team discovered that the individuals showing this metabolic decline already show signs of neuronal injury. “This is a particularly important finding because neuronal death is irreversible”, said Dr. Cortés Canteli, a neuroscientist at the CNIC and a Miguel Servet fellow at the Fundación Jiménez Díaz Health Research Institute.
The CNIC team also discovered that the progression of subclinical atherosclerosis in the carotid arteries over 5 years is linked to a metabolic decline in brain regions vulnerable to Alzheimer’s disease, in addition to the effect of cardiovascular risk factors. “These results provide yet another demonstration that the detection of subclinical atherosclerosis with imaging techniques provides highly relevant information,” said Dr. Fuster, who is the principal investigator on the PESA study. “The interaction between the brain and the heart is a fascinating topic, and with this study we have seen that this relationship begins much earlier than was thought.”
The scientists conclude that considering these results, “carotid screening has great potential to identify individuals at risk of cerebral alterations and cognitive decline in the future.” In the published article they write, “this work could have important implications for clinical practice since it supports the implementation of primary cardiovascular prevention strategies early in life as a valuable approach for a healthy cerebral longevity.”
“Although we still don’t know what impact this decline in cerebral metabolism has on cognitive function, the detection of neuronal injury in these individuals shows that the earlier we start to control cardiovascular risk factors, the better it will be for our brain,” concluded Dr. Cortés Canteli.
The PESA study is equally financed by the CNIC and Banco Santander. The PESA study also receives funding from the Instituto de Salud Carlos III (ISCIII, PI15/02019 & PI20/00819), the European Regional Development Fund (ERDF - A Way to Build Europe) and the European Social Fund (ESF - Investing in Your Future). The present study received funding from the BrightFocus Foundation and a Leonardo Investigadores y Creadores Culturales award. The CNIC is supported by the ISCIII, the Ministerio de Ciencia e Investigación (MCIN), and the Pro-CNIC Fundacion. The study included the participation of investigators in three Spanish research networks (CIBER): Cardiovascular Disease (CiberCV); Frailty and Healthy Aging (CiberFES); and Bioengineering, Biomaterials, and Nanomedicine (Ciber-BBN).
DIABETES CARE
Obesity, high blood pressure, and lipid imbalance trigger a progressive loss of energy generation capacity in the heart
Cardiovascular disease and dementia frequently occur together in elderly people. Nevertheless, few longitudinal studies have examined how atherosclerosis and its associated risk factors affect brain health from middle age. Now, a new study by scientists at CNIC provides new data on this relationship; the results confirm the importance of controlling traditional cardiovascular risk factors, such as hypertension, cholesterol, diabetes, smoking, and a sedentary lifestyle, not only to preserve cardiovascular health, but also to prevent Alzheimer’s disease and other dementias.
Published in The Lancet Healthy Longevity, the CNIC study shows that atherosclerosis—the accumulation of fatty deposits in the arteries—and its associated risk factors, in addition to being the main cause of cardiovascular disease, are also implicated in the cerebral alterations typically found in Alzheimer’s disease, the most frequent cause of dementia.
According to CNIC General Director Dr. Valentín Fuster, an author on the study, the new findings are important because they open up the possibility of treating a modifiable disorder (cardiovascular disease) to prevent the development of a disease for which there is currently no curative treatment (dementia). “The sooner we act to control cardiovascular risk factors, the better it is for our brain health,” said Dr. Fuster.
Cardiovascular risk factors are important for preventing a heart attack,” continued Dr. Fuster. “Nevertheless, the additional information linking the same risk factors to a decline in brain health could further increase awareness of the need to acquire healthy habits from the earliest life stages.”
In 2021, CNIC scientists discovered that the presence of cardiovascular risk factors and subclinical (presymptomatic) atherosclerosis in the carotid arteries (the arteries that supply the brain) was associated with lower glucose metabolism in the brains of apparently healthy 50-year-old participants in the PESA-CNIC-Santander study (Cortés-Canteli & Gispert et al. JACC. 2021). Glucose metabolism in the brain is considered an indicator of brain health.
The PESA-CNIC-Santander, study, directed by Dr. Fuster, is a prospective study that includes more than 4000 asymptomatic middle-aged participants who have been exhaustively assessed for the presence and progression of subclinical atherosclerosis since 2010.
Dr. Fuster’s team, led by Drs. Marta Cortés Canteli and Juan Domingo Gispert, have continued to monitor the cerebral health of these participants over 5 years. Their research shows that individuals who maintained a high cardiovascular risk throughout this period had a more pronounced reduction in cerebral glucose metabolism, detected using imaging techniques such as positron emission tomography (PET).
“In participants with a sustained high cardiovascular risk, the decline in cerebral metabolism was three times greater than in participants who maintained a low cardiovascular risk,” commented Catarina Tristão-Pereira, first author on the study and INPhINIT fellow.
Glucose is the main energy source for neurons and other brain cells. “If there is a sustained decline in cerebral glucose consumption over several years, this may limit the brain ability to withstand neurodegenerative or cerebrovascular diseases in the future,” explained Dr. Gispert, an expert in neuroimaging at the CNIC and Barcelonaßeta Research Center.
Through a collaboration with Drs Henrik Zetterberg and Kaj Blennow, world experts in the identification of new blood biomarkers at the University of Gothenburg in Sweden, the CNIC team discovered that the individuals showing this metabolic decline already show signs of neuronal injury. “This is a particularly important finding because neuronal death is irreversible”, said Dr. Cortés Canteli, a neuroscientist at the CNIC and a Miguel Servet fellow at the Fundación Jiménez Díaz Health Research Institute.
The CNIC team also discovered that the progression of subclinical atherosclerosis in the carotid arteries over 5 years is linked to a metabolic decline in brain regions vulnerable to Alzheimer’s disease, in addition to the effect of cardiovascular risk factors. “These results provide yet another demonstration that the detection of subclinical atherosclerosis with imaging techniques provides highly relevant information,” said Dr. Fuster, who is the principal investigator on the PESA study. “The interaction between the brain and the heart is a fascinating topic, and with this study we have seen that this relationship begins much earlier than was thought.”
The scientists conclude that considering these results, “carotid screening has great potential to identify individuals at risk of cerebral alterations and cognitive decline in the future.” In the published article they write, “this work could have important implications for clinical practice since it supports the implementation of primary cardiovascular prevention strategies early in life as a valuable approach for a healthy cerebral longevity.”
“Although we still don’t know what impact this decline in cerebral metabolism has on cognitive function, the detection of neuronal injury in these individuals shows that the earlier we start to control cardiovascular risk factors, the better it will be for our brain,” concluded Dr. Cortés Canteli.
The PESA study is equally financed by the CNIC and Banco Santander. The PESA study also receives funding from the Instituto de Salud Carlos III (ISCIII, PI15/02019 & PI20/00819), the European Regional Development Fund (ERDF - A Way to Build Europe) and the European Social Fund (ESF - Investing in Your Future). The present study received funding from the BrightFocus Foundation and a Leonardo Investigadores y Creadores Culturales award. The CNIC is supported by the ISCIII, the Ministerio de Ciencia e Investigación (MCIN), and the Pro-CNIC Fundacion. The study included the participation of investigators in three Spanish research networks (CIBER): Cardiovascular Disease (CiberCV); Frailty and Healthy Aging (CiberFES); and Bioengineering, Biomaterials, and Nanomedicine (Ciber-BBN).
NATURE COMMUNICATIONS
Spanish scientists discover a possible treatment for a heart condition that causes an estimated 20% of sudden deaths among athletes

A team of researchers at CNIC has discovered a possible treatment for a disease that causes sudden death in athletes. Arrhythmogenic cardiomyopathy is an incurable disease of the heart muscle that is responsible for an estimated 20% of sudden deaths recorded in professional athletes.
The CNIC team, led by Dr. Juan A. Bernal, who heads the CNIC Viral Vectors Unit, has discovered a treatment that restores the contractile capacity of the myocardium in animal models of arrhythmogenic cardiomyopathy.
The researchers investigated mutations in the protein plakofilin-2 (PKP2) that cause arrhythmogenic cardiomyopathy and discovered a previously unknown function of PKP2, a protein that controls the functional connections between myocytes in the heart. The results are published in the journal Nature Communications.
Arrhythmogenic cardiomyopathy has two major clinical manifestations, cardiac contraction defects and malign ventricular arrhythmias. These can cause loss of consciousness and even sudden death, often linked to episodes of intense physical activity.
Lead investigator Juan A. Bernal explained that the study “focuses on the contraction defect in this disease. Through this approach, we have been able to discover the reason the myocytes do not contract sufficiently and identify a way to solve this problem. Understanding the mechanisms of this disease is an essential step toward developing new treatments.”
Scientists have reported more than 350 mutations in PKP2, “but we don’t know which ones cause disease or how aggressive they are,” said Bernal. “What we have known for several years is that intense physical exercise accelerates the development of the disease.”
While there is still a long way to go to fully define the molecular basis of arrhythmogenic cardiomyopathy, the current study identifies a group of mutations that are consistently associated with severe contractile problems that respond to treatment with an activator of myosin regulators called 4-hydrozyacetophenone (4-HAP).
“Our results provide proof of concept that the generation of a complete atlas of all PKP2 mutations can help to stratify the risk of patients with arrhythmogenic cardiomyopathy so that sudden deaths, like the famous case of Sevilla FC player Antonio Puerta, who died in 2007, can be prevented in the future,” said Dr. Nieves García-Quintáns, the first author on the study.
“Our quest for treatments for this terrible disease that preys on young athletes would have been impossible without the public–private funding from the CNIC and the support of the Ministerio de Ciencia, Innovación y Universidades, the “la Caixa” Foundation, and Real Madrid Graduate School - Universidad Europea. We are also keen to establish contacts with other clubs that might be interested in supporting our research,” added Dr. Bernal.
The full Imaris analysis was conducted by the CNIC Microscopy and Dynamic Imaging Unit (ICTS-ReDib), which is cofinanced by Ministerio de Ciencia e Innovación (MCIN).
NATURE COMMUNICATIONS
Spanish scientists identify changes in dendritic cells during the immune response with promising implications for vaccine design
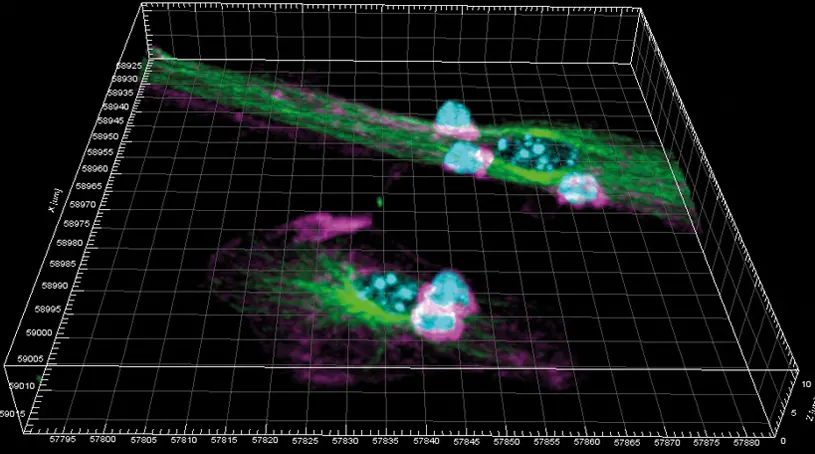
A team of scientists at the(CNIC and Hospital de la Princesa-UAM in Madrid have discovered that dendritic cells, an important cell type in the immune response to viral and bacterial infections, are profoundly changed by their involvement in this process in ways that were previously unknown.
In a study published in Nature Communications, the scientific team led by Prof. Francisco Sánchez-Madrid provide invaluable information about the mechanisms involved in the immune response to pathogens and point the way to the design of novel vaccines against future pandemics.
Study first author Dr. Diego Calzada Fraile explained that during the immune response to infection, “dendritic cells, which can be likened to sentries stationed in our tissues, are able to detect the pathogen, ingest or absorb it, and degrade it into small fragments which they present on their surface to lymphocytes, the effector cells of the immune system that can then recognize the pathogen and mount a specific response.”
This process of antigen presentation requires the dendritic cell and its lymphocyte partner to come into close contact through the formation of a dynamic structure called an immune synapse, through which these cells can exchange information.
The study shows that the formation of the immune synapse not only activates the lymphocyte, as was well known, but also triggers profound changes within the post-synaptic dendritic cell.
The scientists describe important changes in the protein content of the post-synaptic dendritic cell. In addition, as Dr. Calzada Fraile explained, the study identifies “increased lipid peroxidation in dendritic cells as key mechanism for increasing the efficiency of antigen presentation to cytotoxic CD8 lymphocytes.”
Using a mouse model developed by collaborators at the University of Padova in Italy, the scientists were able to analyze and manipulate post-synaptic dendritic cells that had interacted with T lymphocytes during an immune response in vivo triggered by vaccination with an antigen formulated with alum, the most widely used adjuvant used in human vaccines.
As Prof. Francisco Sánchez-Madrid explained, “Post-synaptic dendritic cells are responsible for the generation of specific CD8 lymphocytes in response to a vaccine, and thus present an attractive target for interventions aimed at stimulating CD8 lymphocyte responses during vaccination.”
According to Prof. Francisco Sánchez-Madrid, the importance of this finding lies in the fact that “the effectiveness of a vaccine depends on its ability to trigger a strong CD8 response, because this determines the level of protection against a wide range of infections.”
The potent ability of post-synaptic dendritic cells to activate cytotoxic CD8 T lymohocytes suggests that they have therapeutic potential as a type of vaccine that could be administered to boost the body’s defenses against bacterial and viral infections.
One of the attractions of such a strategy is that “unlike conventional vaccines, the protection does not depend on the inclusion of material from the pathogenic organism, so this approach could be used to combat unknown pathogens that might emerge and cause a new pandemic.”
The study was partly funded through the la Caixa Health Research grant HR17-00016 (FS-M) and a postgraduate INPhINIT Retaining Fellowship from the la Caixa Foundation (DC-F).
CIRCULATION RESEARCH
CNIC scientists identify the crucial role of the protein neuregulin-1 in heart development
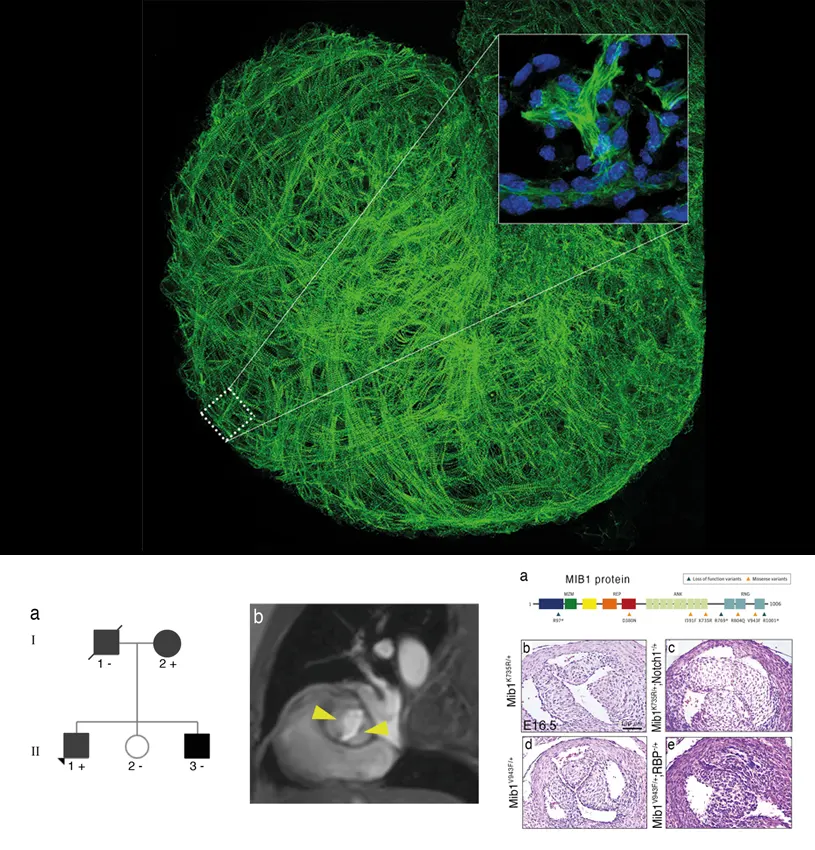
A team of scientists at the CNIC has identified a key element of the machinery of heart formation. In a study published in the journal Circulation Research, researchers led by Dr. José Luis de la Pompa reveal the essential role of the protein neuregulin-1 (Nrg1) in the intricate transformation of the heart from its delicate primordial structure into a powerful pumping organ.
The findings not only highlight the pathways through which the human heart forms, but also suggest important directions for future medical advances.
The heart is the body’s engine and is composed of a number of specialized parts. One of these essential components is the ventricles, the heart chambers whose muscular contractions make the ‘lub’ and ‘dub’ sounds of the heartbeat and are responsible for pumping blood to the lungs and around the body throughout life.
Scientists have long been fascinated by how these chambers, initially structured in delicate protrusions called trabeculae, grow, and mature into the robust structures that keep the heart beating.
Study first author Joaquín Grego-Bessa explained that trabeculae can be likened to a scaffolding that supports the heart as it grows: “We can think of trabeculae as small, primitive projections that form the foundation of the heart.” Understanding how these structures develop into the mature ventricles is not just a fascinating biological question; it also has immense potential to advance regenerative medicine, offering new perspectives on heart conditions and their treatment.
The new study identifies a crucial actor in this process: Nrg1, a signalling protein that guides the trabeculae formation. Nevertheless, mystery still surrounded the intricate mechanisms through which Nrg1 operates and its role in the maturation of the heart wall.
To resolve this mystery, the CNIC team carried out experiments using advanced imaging techniques, genetic analysis, and biochemical studies in mice. Manipulation of the amount of Nrg1 expressed in cardiac cells revealed fascinating patterns.
“The experiments revealed that Nrg1 orchestrates a symphony of events within cardiac cells,” explained Donal MacGrogan, joint lead author on the study. Through these actions, he continued, “Nrg1 coordinates the division of cardiac cells to form trabeculae, ensuring that these structures grow in the appropriate orientation.”
The scientists view Nrg1 as the guiding hand that molds the architecture of the heart. Commenting on the results, Donal MacGrogan said that “when Nrg1 levels were altered, the cardiac cells changed their behaviour, and this resulted in structural and functional irregularities. These changes are like a stumbling block in the growth of the heart, potentially leading to cardiac problems in affected individuals in the future.”
José Luis de la Pompa emphasized that understanding how Nrg1 functions is more than an intellectual exercise and “could provide a route to revolutionary treatments in the future. Decoding the language of heart development could unblock new strategies for the repair of cardiac injury. This research not only deepens our understanding of how the heart grows and functions, but also provides hope for those engaged in the fight against heart diseases.”
The study was supported by the Ministerio de Ciencia e Innovación, CIBER CV, Fundación BBVA, Fundació La Marató de TV3, Sociedad Española de Cardiología and Programa Atracción de Talento de la Comunidad de Madrid.
JACC
Younger people are more vulnerable to the effects of cardiovascular risk associated with high blood cholesterol and hypertension
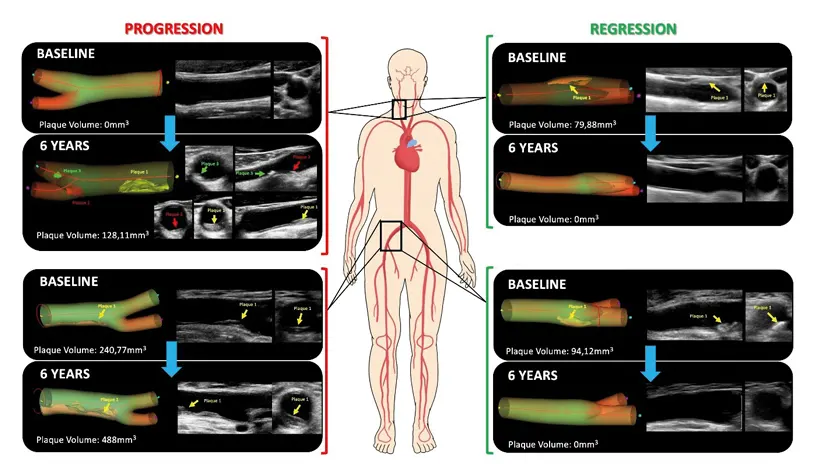
Young people may be more susceptible to the effects of the risk factors for developing atherosclerosis. According to a study carried out at the CNIC, younger people are especially vulnerable to the damaging effects of elevated blood cholesterol and hypertension, two of the major modifiable cardiovascular risk factors.
These findings, published in the Journal of the American College of Cardiology, underline the need to implement aggressive control of cardiovascular risk factors at younger ages, requiring a change in primary prevention strategies to include “surveillance of subclinical atherosclerosis and early cardiovascular risk factor control.”
Subclinical aterosclerosis often progresses in middle aged individuals, especially if LDL-cholesterol levels and blood pressure are even mildly or moderately elevated. Medical professionals and the public need to be aware that atherosclerosis progression can be stopped if risk factors are managed agressively from an early age.
“Screening for subclinical atherosclerosis from an early age together with aggressive risk-factor control could help to reduce the global burden of cardiovascular disease,” said D. Valentín Fuster, CNIC General Director and Physician-in-Chief at Mount Sinai Medical Center in New York.
Dr. Borja Ibáñez, CNIC Scientific Director, a cardiologist at Hospital Universitario Fundación Jiménez Díaz, and a member of the CIBERCV cardiovascular research network in Spain explained that “in this study, we show that moderate increases in blood pressure and cholesterol have a much more pronounced impact on atherosclerosis progression in younger people.”
Very few studies have investigated the progression of silent atherosclerosis in people who are completely free of symptoms, whether they are young or in apparently healthy middle-age, and how this disease progresses throughout life.
The PESA-CNIC-Santander study (Progression of Early Subclinical Atherosclerosis) was launched in 2009 and is a close collaboration between the CNIC and Santander Bank.
The current findings have important implications for cardiovascular prevention and personalized medicine. The study shows that the control of risk factors (principally elevated cholesterol and hypertension) should begin early in life when the arteries are more vulnerable to the effects of these risk factors.
And as Dr. Borja Ibáñez emphasized, “these results point the way to personalized approaches that use imaging technology to monitor the presence and progression of silent atherosclerosis and guide the intensity of risk-factor control.
Cardiologist Guiomar Mendieta, the first author on the study, added that “the other key finding of this study is that atherosclerosis, previously believed to be irreversible, can disappear if risk factors are controlled from an early stage.”
“These findings are the outcome of the exhaustive collection of imaging and biochemical data over 6 years, combined with an innovative statistical analysis,” explained Dr. Mendieta, who joined the CNIC through the CARDIOJOVEN SEC-CNIC training program, a joint initiative of the CNIC and the Spanish Society of Cardiology.
The CNIC investigators received funding from the European Commission (ERC-CoG 819775 and H2020-HEALTH 945118), the Spanish Ministry of Science and Innovation (PID2019-110369RB-I00), and the Community of Madrid regional government (P2022/BMD-7403, RENIM-CM).
NATURE CARDIOVASCULAR RESEARCH
Spanish scientists discover a promising therapeutic target for cardiac arrhythmias
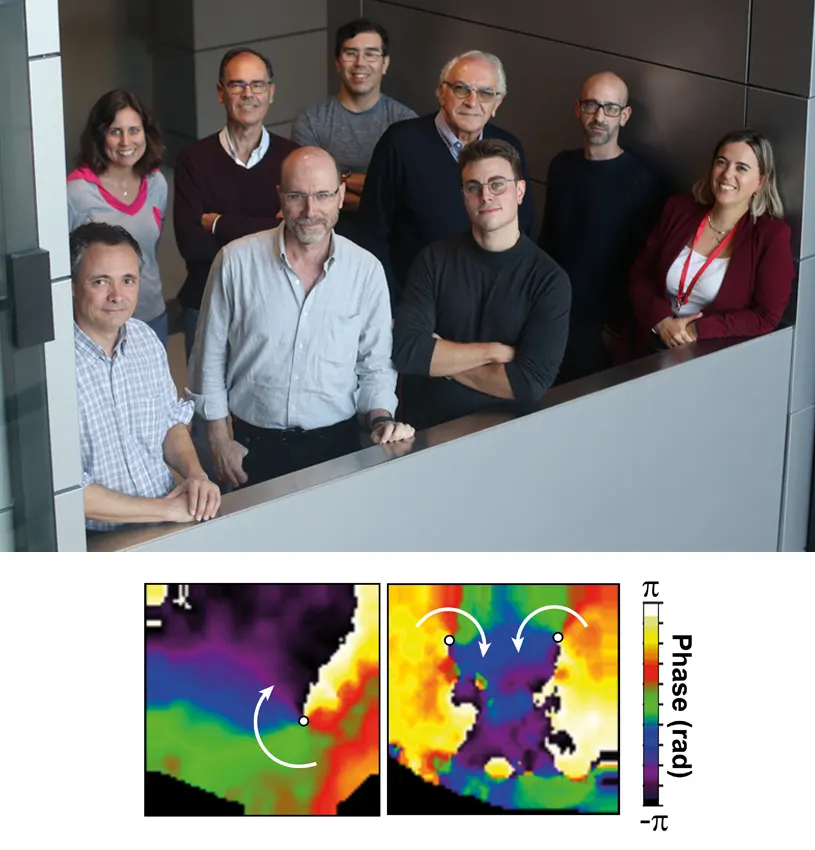
A study led by Guadalupe Sabio and José Jalife at the CNIC in Madrid has identified a new signaling mechanism implicated in the development of ventricular fibrillation, a type of arrhythmia, or irregular heartbeat. The study findings, published in the journal ‘Nature Cardiovascular Research, offer promise of future treatment options for this life threatening condition.
Ventricular fibrillation is the most frequent cause of sudden cardiac death. Although aging is an established risk factor for the development of cardiac arrhythmia, the mechansims underlying this connection have been hard to pin down, hindering progress toward the development of specific treatments.
Working with animal models, the CNIC team discovered a connection between the development of ventricular fibrillation and the activation of two key signaling proteins, the stress kinases p38γ and p38δ. The link with these enzymes was independent of the sex of the animals.
The scientists found that p38γ and p38δ phosphorylate a receptor called ryanodine receptor 2 and another protein called SAP97, resulting in a mislocalization of the potassium ion channel Kv4.3. These molecular changes lead to premature ventricular activation and an increased susceptibility to ventricular fibrillation.
The study findings identify a promising therapeutic target for the development of new strategies to prevent sustained ventricular fibrillation and provide protection against this serious condition.
El estudio ha sido financiado por un Proyecto Intramural CNIC Severo Ochoa, un proyecto IMPACT-2021 (PMP21/00057) del Instituto de Salud Carlos III (ISCIII), el Ministerio de Ciencia e Innovación (PDC2021-121147-I00 y PID2019-104399RB-I00), y la Unión Europea (FEDER/FSE), “Una manera de hacer Europa”/ “El FSE invierte en tu futuro”/ Next Generation EU. Además, ha recibido ayudas de la American Heart Association, la Fundació Marató TV3 y la Fundación Bancaria “la Caixa”.
CIRCULATION RESEARCH
CNIC scientists identify the molecular mechanisms controlling the genes involved in proper formation of the heart valves

A team of researchers at CNIC has identified the molecular mechanisms that control the activity of genes involved in both the correct formation of the heart valves and the prevention of their subsequent calcification.
The findings, published in the journal Circulation Research, not only highlight the pathways through which the human heart forms, but also offer clues for future medical advances. Study coordinator Dr. José Luis de la Pompa, who heads the Intercellular Signaling in Cardiovascular Development and Disease Laboratory at the CNIC, explained that, “by understanding these fundamental processes, scientists are one step closer to solving the mysteries of the human heart and its pathologies”.
The heart is the body’s engine, and its correct function depends on the interaction of specialized components. One of these components is the endocardium, the single layer of cells that lines the inside of the heart, insulating the heart muscle from the blood it pumps throughout the body.
“But this is not its only function,” explained lead author and study co-coordinator Dr. Luis Luna Zurita: “This inner lining of the heart produces molecular signals that ensure the correct organization and function of the heart during embryonic development.”
For example, the endocardium plays an essential role in the formation of the heart valves.
“In response to different signals, cells in specific regions of the endocardium transform, acquire invasive properties, and colonize a territory beneath the myocardium, gradually forming structures called the valve primordia, which undergo progressive modeling to generate the mature adult heart valves,” said Dr. Luna Zurita.
Heart valves are crucial structures that regulate the one-way blood flow in the heart. One of the four heart valves is the aortic valve, located at the junction between the left ventricle and the aorta. In approximately 1% of the population, this valve has only two leaflets (bicuspid aortic valve, BAV) instead of the usual three (triscuspid aortic valve, TAV).
For example, the endocardium plays an essential role in the formation of the heart valves.
“In response to different signals, cells in specific regions of the endocardium transform, acquire invasive properties, and colonize a territory beneath the myocardium, gradually forming structures called the valve primordia, which undergo progressive modeling to generate the mature adult heart valves,” said Dr. Luna Zurita.
Heart valves are crucial structures that regulate the one-way blood flow in the heart. One of the four heart valves is the aortic valve, located at the junction between the left ventricle and the aorta. In approximately 1% of the population, this valve has only two leaflets (bicuspid aortic valve, BAV) instead of the usual three (triscuspid aortic valve, TAV).
“This bicuspid valve is more prone to deteriorate and gives rise to a number cardiovascular pathologies”, explained Dr. de la Pompa. One of these is valve calcification, which is frequent in these patients but also affects individuals with no apparent valve defect.
The accumulation of calcium deposits on the aortic valve affect its flexibility and function. One of the key consequences of calcification is valve stenosis, which restricts blood flow and compromises the heart’s pumping action, resulting in the need for surgery to replace the calcified valve.
Studies in experimental models have established that many important steps in cardiovascular development are regulated by the Notch signaling pathway. Disruption of this highly conserved pathway causes both valve defects and valve calcification.
Notch acts on the endocardium at the earliest stages of development and during adult life. Mutations affecting different Notch pathway components are implicated in the formation of a BAV.
By carefully manipulating Notch pathway activity in isolated embryonic endocardial cells, Dr. de la Pompa’s team were able to identify the gene programs activated and inhibited by this pathway in the endocardium, distinguishing between an early and a late response.
To pursue their analysis further, the researchers needed to incorporate large databases from multiple sources. “Big data is becoming increasingly pivotal in numerous sectors, not least in biomedical research,” said Dr. Luna Zurita.
After creating a list of active genes in embryonic endocardial cells, Dr. Luna Zurita compared this with information on genetic programs in the embryonic and adult heart already described by Dr. de la Pompa’s lab. The comparison thus provided insight into valve formation during embryonic development and the calcification of adult valves, enabling the identification of key genes whose suppression disrupts normal valve formation in the embryo, is strongly associated with calcification in adulthood, or adversely impacts both processes.
The research also identified a set of regulatory regions that act on these genes. Dr. Luna Zurita explained that genes encoding proteins make up just 1% of the DNA in a mammalian genome. The regulatory regions are found in the remaining 99% and are responsible for a gene being expressed in a specific organ at a specific time. “This implies that, while identifying changes in gene activity is obviously essential for understanding and treating disease, pinpointing the location of these genes’ regulatory regions is just as crucial. By analyzing changes in DNA compaction, we identified the regions that open or close in response to Notch pathway activity.
Bioinformatics analysis and the integration of the databases from previous studies allowed the researchers to identify those DNA regions that likely control the expression of the genes implicated in heart-valve formation and maintenance. In addition, this analysis provided crucial information about the molecules and signaling pathways responsible for this control.
One of the pathways identified was the Hippo signaling pathway, an extensively researched pathway that plays a crucial role in the control of cell division and organ size. “Our analysis revealed for the first time that cooperation between the Notch and Hippo pathways is required for the correct participation of the endocardium in valve formation,” concluded Dr. de la Pompa.













