Science Advances: Spanish scientists identify the role of the CCT protein in the control of immune-synapse formation
A new study published in Science Advances identifies the role of the cytosolic chaperone protein CCT in the reorganization of the cytoskeleton during the formation of an immune synapse
The activation of T lymphocytes requires their interaction with antigen presenting cells. The contact between the surfaces of these two cell types produces a structure called an immune synapse, whose formation and activity determine the strength of lymphocyte activation and the subsequent immune response.
Formation of the immune synapse is a highly dynamic process, requiring the reorganization of the cytoskeleton and the centrosome, the tightly regulated organelle that controls the arrangement of microtubles at each phase of the cell cycle.
Now, research teams led by Francisco Sánchez-Madrid and Noa Martín at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) and the Instituto de Investigación Sanitaria del Hospital Universitario de La Princesa (IIS Princesa) and by José María Valpuesta at the Centro Nacional de Biotecnología del Consejo Superior de Investigaciones Científicas (CNB-CSIC) have identified the essential role played in this process by the cytosolic chaperone protein CCT. The study, published in the journal Science Advances, describes how CCT controls changes in the arrangement of the centrosome and mitochondria (the organelles that produce energy within the cell) by regulating the production of the cytoskeletal components α- and β-tubulin.
Javier Chichón, a CNB-CSIC researcher and one of the first authors on the study, explained that “CCT is a protein complex involved in the correct folding of proteins with important roles in many cell processes. Two key CCT substrates are actin and tubulin, two essential protein components of the cytoskeleton.” The new study demonstrates the importance of CCT in processes that critically depend on cell dynamism
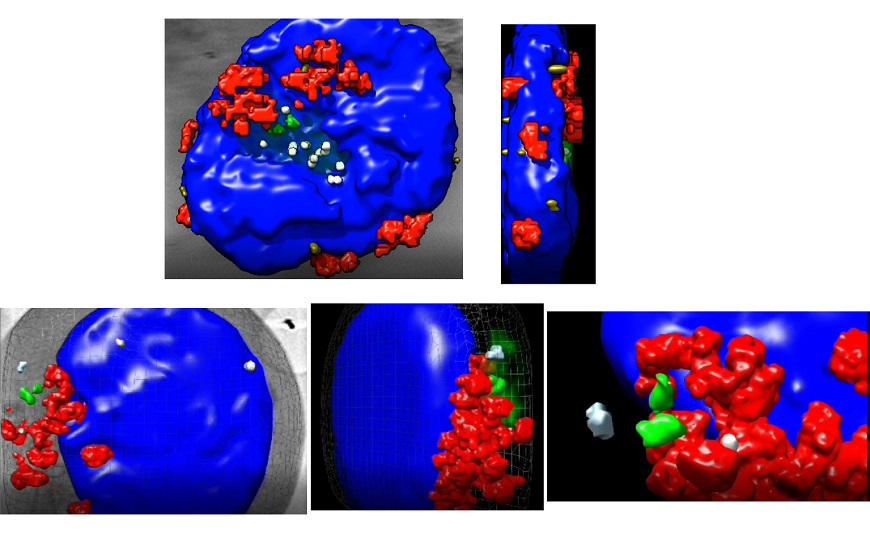
The researchers used cutting edge X-ray cryotomography technology for 3D cell reconstruction, performed in the MISTRAL beamline of the ALBA synchrotron in Cerdanyola del Vallès, Barcelona. Using this technology, the scientists were able to observe the relative movement of the two centriole components of the centrosome and changes in the arrangement of mitochondria and microtubules. This technique, based on synchrotron light, allows cells to be observed in their entirety, without no need for sectioning, in conditions very close to nature. Apart from ALBA, this technology is available in only three other synchrotrons in the world, located in the UK, Germany, and the USA.
The research team also included scientists from the University of Cantabria and the Centre for Genomic Regulation in Barcelona.
Noa Martín, a first author on the study and a researcher at the IIS Princesa and the CNIC, said: “We now know that CCT is responsible for this change, that it exercises its control by regulating the de novo synthesis of α- and β tubulin and the post-translational modifications necessary for microtubule assembly during the formation of the immune synapse, and that these actions regulate cell metabolism.”
This finding opens the path to the design of strategies aimed at blocking CCT for the treatment of autoimmune diseases that involve hyperactivation of lymphoid cells.