PNAS: CNIC scientists identify the key cell type for strategies to prevent atherosclerosis in progeria syndrome
The elimination of progerin from vascular smooth muscle cells, but not from endothelial cells, prevents the atherosclerosis associated with Hutchinson-Gilford progeria syndrome (HGPS)
Hutchinson-Gilford progeria syndrome (HGPS) is an extremely rare genetic disease that affects just 1 in every 20 million people; it is estimated that fewer than 400 children in the world have the disease. HGPS is characterized by accelerated aging, severe atherosclerosis, and premature death at an average age of about 15 years. Although people with HGPS do not normally have conventional cardiovascular risk factors (hypercholesterolemia, obesity, smoking, etc.), most patients die from the complications of atherosclerosis: myocardial infarction, stroke, or heart failure. HGPS currently has no cure, and there is thus an urgent need for new treatments to prevent the atherosclerosis and other vascular alterations associated with the disease and to increase life expectancy.
HGPS is caused by a mutation in the LMNA gene that leads to the expression of progerin, a mutated version of the nuclear protein lamin A that induces numerous defects in cells and body systems.
Recent studies in animal models of HGPS have demonstrated that the mutation can be corrected using gene-editing technology, and that the resulting elimination of progerin and restoration of lamin A expression reduces the symptoms of HGPS and increases life expectancy.
The optimization of gene therapy for possible treatment of HGPS patients will require the identification of the cell types in which elimination of progerin produces the most benefit.
To address this challenge, the laboratory headed by Dr. Vicente Andrés at the Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC) in Madrid previously generated the HGPSrev mouse model. The results of this earlier study, published in the journal Circulation , suggested that vascular smooth muscle cells could be a therapeutic target for combating premature atherosclerosis in progeria.
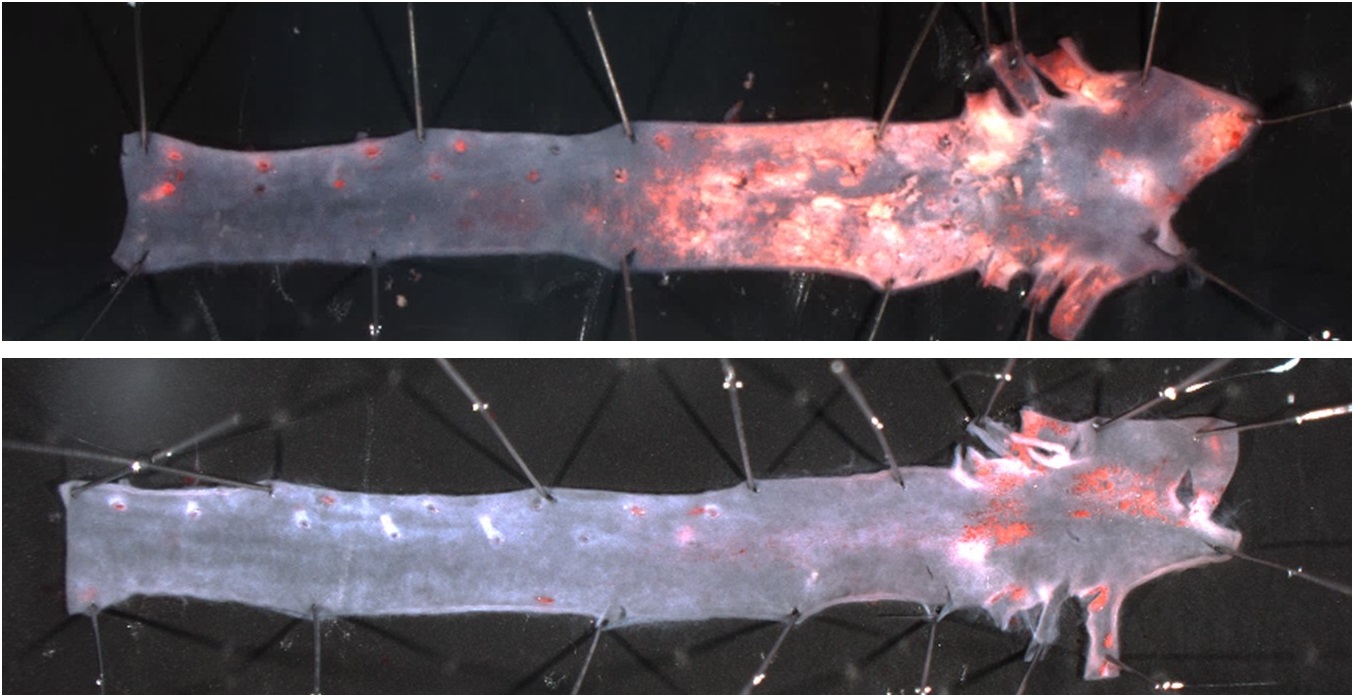
“HGPSrev mice express progerin and lack lamin A in all cells of the body while permitting the elimination of progerin and the restoration of lamin A in specific cell types and at specific disease stages,” explained Dr. Andrés, whose research group also forms part of the Spanish cardiovascular research network (CIBERCV).
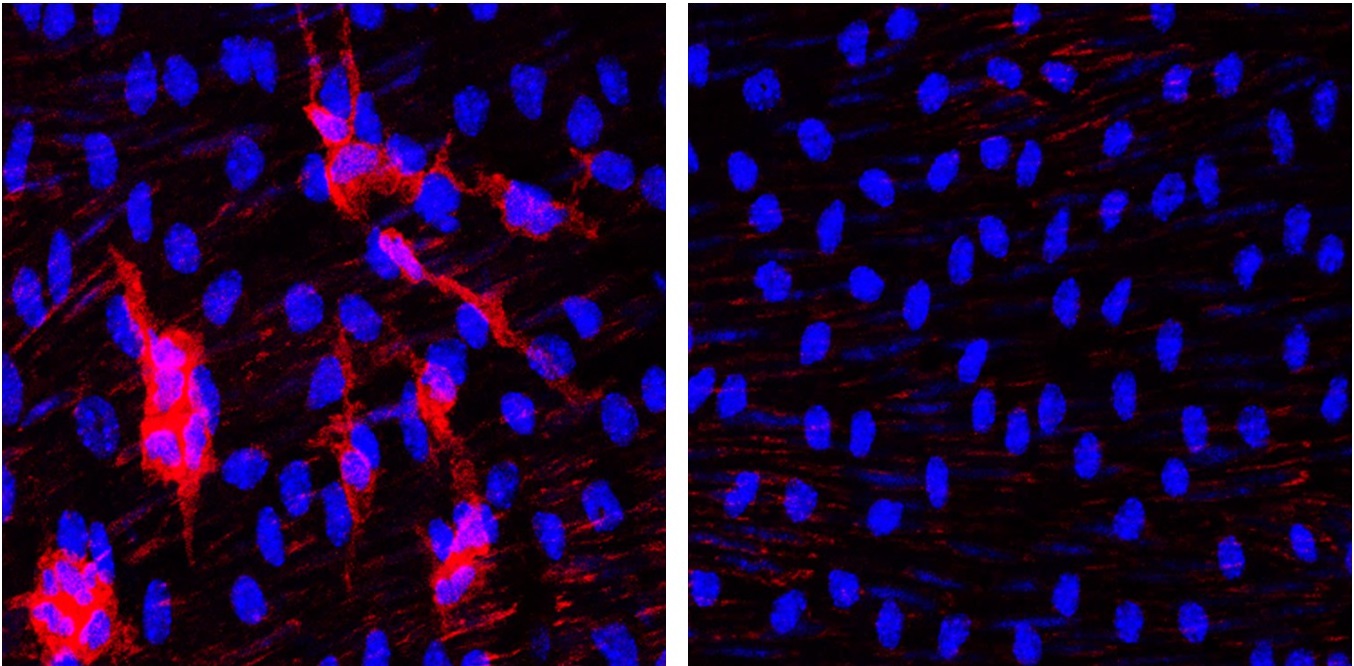
Now, Ignacio Benedicto at the Centro de Investigaciones Biológicas Margarita Salas-CSIC, working with Dr. Andrés’s team and colleagues at the university of Oviedo and Queen Mary University of London, have examined whether atherosclerosis can be avoided in HGPSrev mice by suppressing progerin and restoring lamin A either in endothelial cells or in vascular smooth muscle cells, “two types of cells in the arterial wall that play a central role in the development of atherosclerosis in the general population,” explained Dr. Benedicto. The results of the new study are published in The Proceedings of the National Academy of Sciences.
The researchers discovered that the elimination of progerin from the endothelial cells of HGPSrev mice produced no benefit. These mice developed atherosclerosis, arterial fibrosis, and vascular inflammation and lost weight and died prematurely just the same as mice expressing progerin in all cells of the body. In contrast, HGPSrev mice in which progerin was deleted and lamin A restored in vascular smooth muscle cells did not develop atherosclerosis or other vascular defects and in this respect were indistinguishable from control mice with no progerin expression in any tissue.
“These results suggest that correcting the culprit HGPS mutation in vascular smooth muscle cells could be enough to produce a significant therapeutic benefit,” said Dr. Benedicto.
Dr. Andrés added that “a strategy of this kind would probably require lower doses of gene-editing reagents than needed to eliminate progerin in all tissues, thus increasing the chances of designing effective and safe gene therapy strategies for clinical applications in the future.”
Dr. Andrés also emphasized the importance of investigating rare diseases that affect only a very small number of people. “Considered together, the approximately 7,000 rare diseases known to exist constitute a major health and societal problem that affects 7% of the world population, including 3 million patients in Spain alone. One of the biggest difficulties these patients face is the lack of effective diagnosis and treatment, in large part a consequence of these diseases not receiving sufficient research attention. In the case of progeria, currently there is no cure, and the available palliative therapies are of limited benefit. It is therefore important to continue research in order to gain knowledge about the mechanisms through which progerin accelerates aging in HGPS, develop new treatments, and, eventually, cure this disease.”
The study was funded by theMinisterio de Ciencia, Innovación y Universidades (MICIU)/Agencia Estatal de Investigación (AEI)/10.13039/501100011033 and ERDF/EU (grants PID2022-141211OB-I00 and PID2022-137111OA-I00); the Comunidad Autónoma de Madrid (grants 2017-T1/BMD-5247 and 2021-5A/BMD-20944) cofinanced with European structural and investment funds; RYC2021-033805-I (MICIU/AEI/10.13039/501100011033 and European Union NextGenerationEU/PRTR); the Ministerio de Educación, Cultura y Deporte; Fundación “la Caixa”; and the Wellcome Trust. The CNIC receives institution-level support from the Instituto de Salud Carlos III (ISCIII), the MICIU, and the Pro-CNIC Foundation, and is a Severo Ochoa Center of Excellence (award CEX2020-001041-S funded by MICIU/AEI/10.13039/501100011033).











