Molecular Genetics of Angiogenesis
The vascular system is distributed throughout the entire organism and is very heterogeneous, adapting its structure, function and genetic program to the different organs. The progression of many diseases is dependent on the vascular system, and its transition from a quiescent to an angiogenic state.
The laboratory’s main objective is to obtain a better understanding of how distinct signalling pathways control the different context-dependent behaviours of the diverse cell types that compose the vascular system and that are essential for new blood vessel formation, a process named as angiogenesis. This knowledge will be fundamental to better modulate angiogenesis in growing, damaged, ischemic or cancerous tissues. We are also investigating how blood vessels crosstalk with other adjacent organ cell types, to regulate their homeostasis or response in disease or tissue regeneration settings.
The laboratory has a strong expertise in a wide range of methods, including molecular biology, mouse genetics, vascular phenomics, single cell lineage tracing and high resolution imaging. In the last years the group has put a special effort in generating new genetic tools and mouse lines that allow the understanding of gene function with higher cellular and temporal resolution. ifgMosaic mice enables the induction at any given time point and in any tissue, multispectral and combinatorial genetic mosaics for higher resolution gene function analysis (Pontes-Quero et al., 2017, Cell). In these mice, different cells of the same tissue express different genes that are associated to specific fluorescent proteins, enabling the direct imaging and comparison of distinct cellular phenotypes caused by the single or combinatorial expression of those genes. This is important to understand how individual or a combination of genes regulate the biology of single cells, during a given biological process (Figure 1).
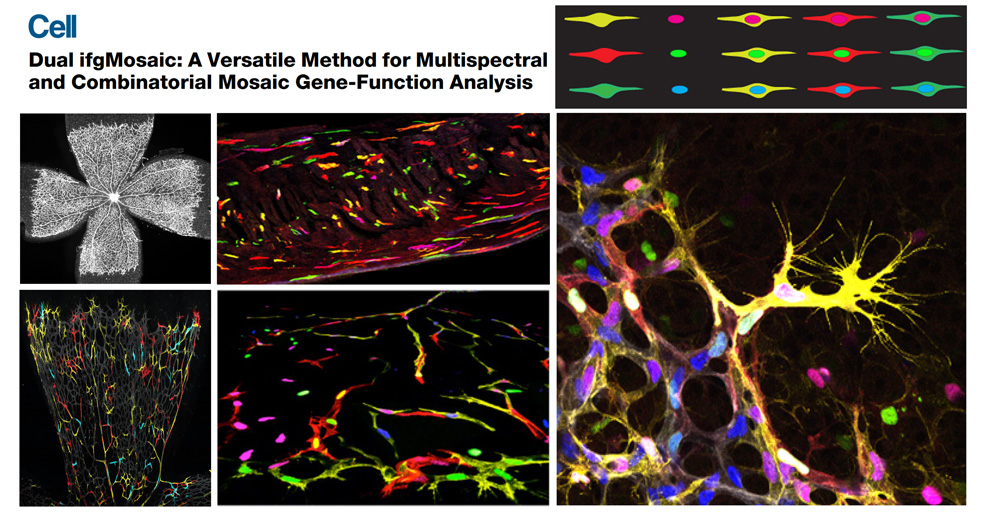
Figure 1. Dual ifgMosaic technology to multispectrally barcode individual cells.
The focus on new technology development allowed the laboratory to achieve a high level of cellular resolution and genetic certainty in cardiovascular biology studies. Using these new models and advanced imaging methods the group has found that induction of vascular endothelial growth factor (VEGF) signalling or inhibition of Notch signalling, strongly arrests angiogenesis (Pontes-Quero et al., 2019 Nature Communications - Figure 2). The effect of pharmacological compounds targeting these pathways varies with the stage of angiogenesis and the vascular context and often leads to paradoxical results. High mitogenic stimulation induced by growth factor signalling, such as VEGF signalling, induces the proliferation of more mature or quiescent vessels, but arrests the proliferation of angiogenic vessels, which are the most relevant for therapeutic angiogenesis. The observed arrest of angiogenesis is due to a bell-shaped dose-response to mitogenic stimulation. At high levels of mitogenic stimulus, endothelial cells migrate and sprout, but do not proliferate, because they activate the expression of cell-cycle checkpoints and inhibitors, such as p21 (Figure 2). Eventually, this affects the sustainable development of the blood vessels and the growth or regeneration of the surrounding tissues.
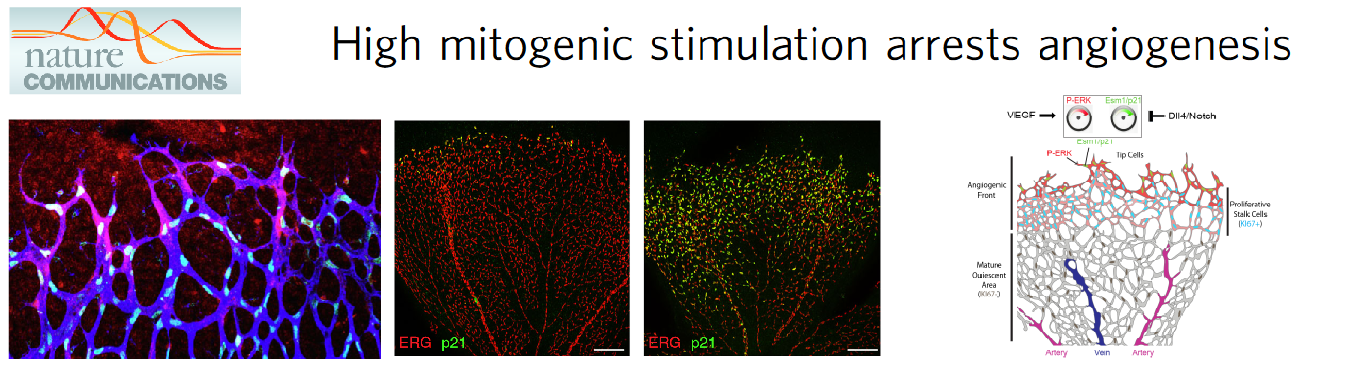
Figure 2. Angiogenesis requires a mitogenic balance regulated by Notch and VEGF signalling.
The group has also given an important contribution to the understanding of how arteries develop (Luo et al., 2021 Nature), knowledge that can be later used to induce effective collateral arterialization in cardiovascular disease. Through the generation and use of novel ratiometric and mosaic functional lineage tracing genetics, the group defined the function of the VEGF and Notch signalling pathways in the proliferation, arteriovenous (AV) differentiation and mobilization of endothelial cells during the process of arterialization (Figure 3). Cells with highest VEGF and Notch signalling in pre-arterial capillaries displayed lower Myc-dependent metabolic and cell-cycle activities, promoting the incorporation of ECs in arteries. Mosaic lineage tracing studies revealed that mutant ECs completely lacking the master regulator of arterialization Notch/Rbpj rarely form arteries; however, cells losing Rbpj and Myc regained the ability to form arteries. These results showed that arterial development does not require the direct induction of an arterial differentiation genetic programme, but rather the timely suppression of endothelial cell-cycle progression and metabolism, a process preceding arterial mobilization and complete differentiation.
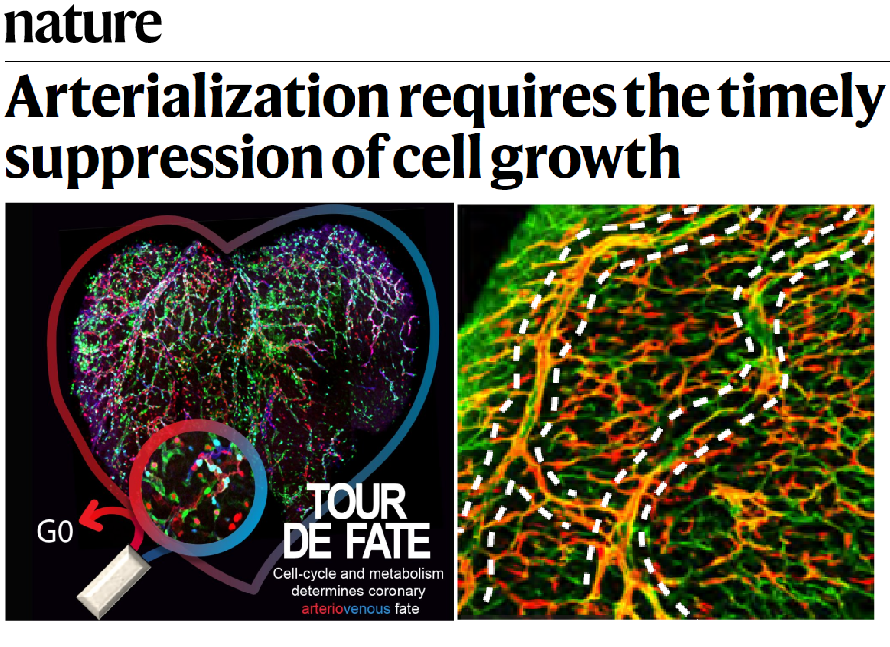
Figure 3. Cell cycle and metabolism determines Arterial-Venous fate.
More recently, the group also found that the toxicity of compounds used to target Notch ligands and receptors in order to modulate angiogenesis in cancer or cardiovascular disease cannot be simply explained by the genetic or transcriptional changes they induce in the vessels (Figure 4). The group found that targeting the Notch ligand Dll4 induced pathologic vascular enlargement and remodelling in contrast to targeting its receptors. This vascular enlargement occurred even when angiogenic genetic programs were silenced, suggesting that these are not responsible for the toxicity and vascular abnormalization observed after the use of pro-angiogenic molecules like anti-Dll4. The observed incongruence between the transcriptional and the vascular pathophysiological cell states (Figure 4) raise questions about the exact mechanisms involved and the general use of single-cell transcriptional or genetic states to describe and predict functional or dysfunctional vascular phenotypes and ultimately organ pathophysiology. These results will also help us to select the most effective and safe way to modulate angiogenesis in ischemic tissues or in cancer, without compromising vascular integrity and homeostasis.
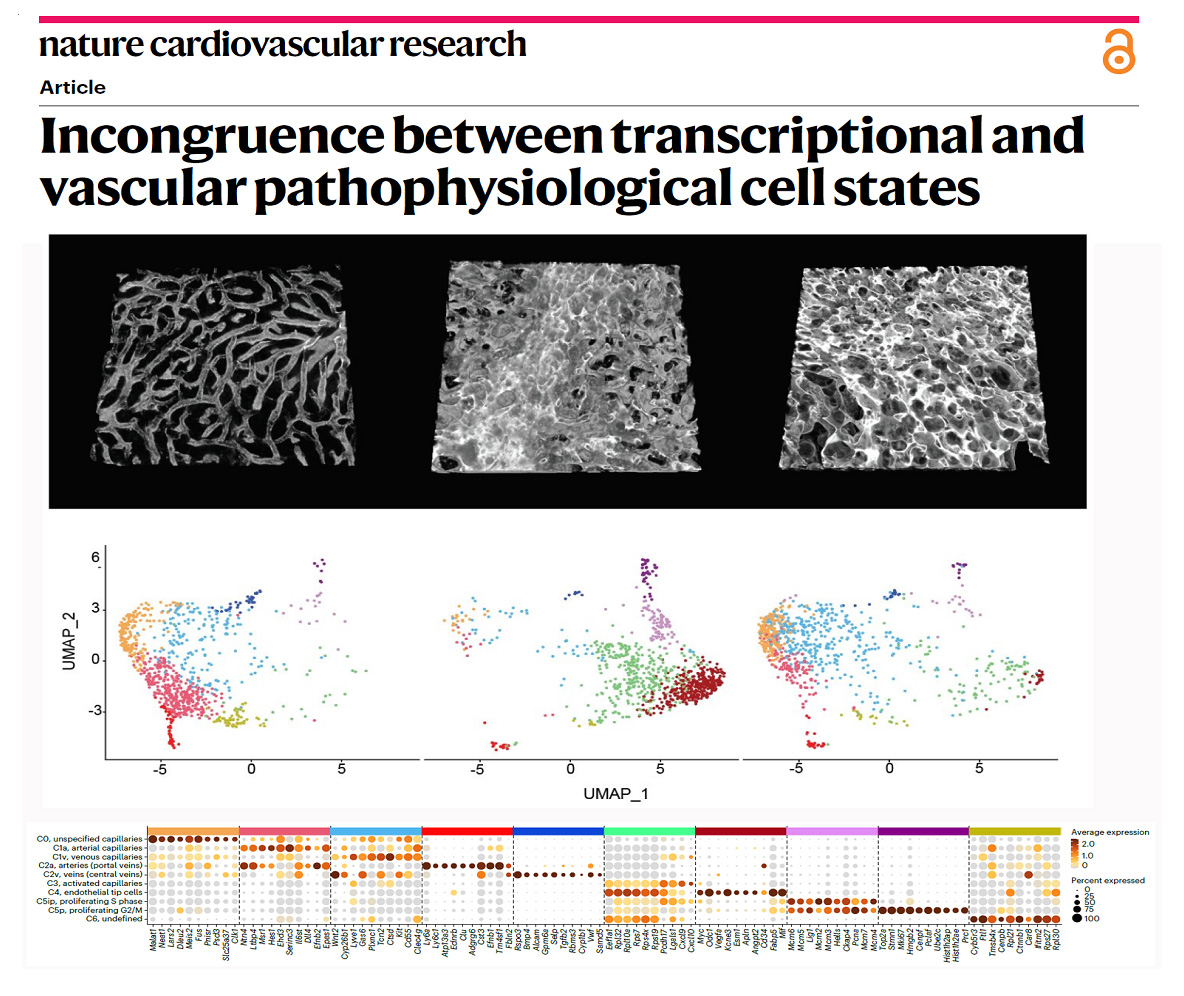
Figure 4 - Transcriptional states often do not correlate with vascular function and pathology.
The group continues to develop genetic technologies of broad relevance for biomedical research. The latest is the new iSuRe-HadCre technology, a new genetic tool essential for effective conditional genetics using mouse models (Garcia-Gonzalez et al., 2024 Nucleic Acids Research). This tool increases the ease, efficiency, and reliability of conditional mutagenesis and gene function analysis (Figure 5).
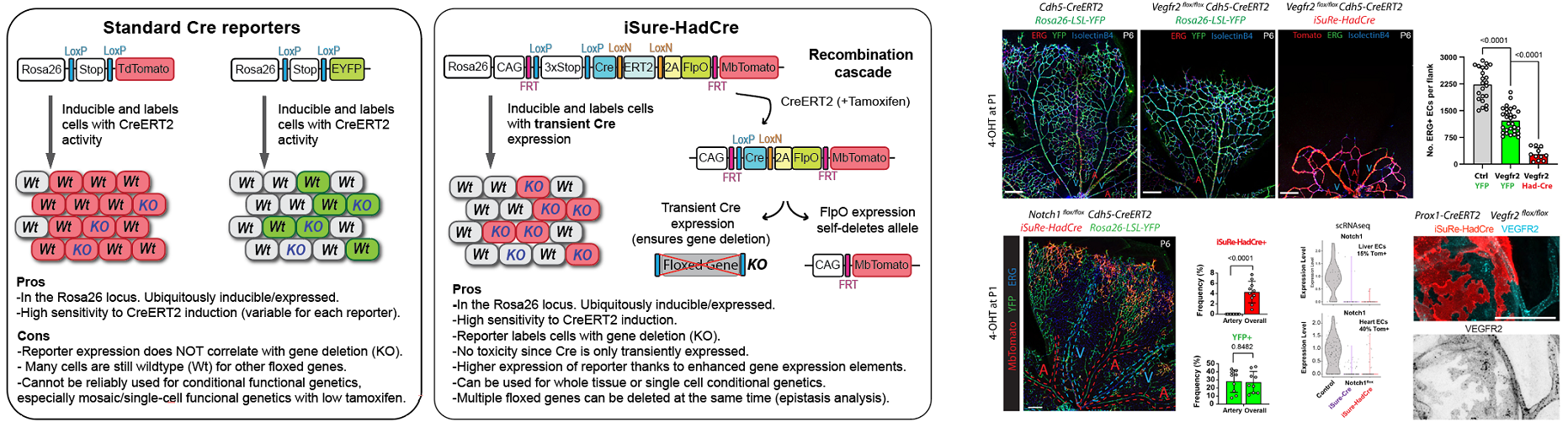
Figure 5. New iSuRe-HadCre technology to ensure conditional genetic deletions in mouse models.
In the next years, the group will continue to develop new genetic technology of broad relevance, in order to be able to analyse gene function with higher accuracy and resolution in vivo. These technologies will allow us to better understand the main genetic mechanisms controlling the biology of blood vessels, not only during tissue development but also in homeostasis or in cardiovascular disease. This knowledge can be later used to effectively regulate angiogenesis and vascular function in diverse physiological and pathological settings (Figure 6).
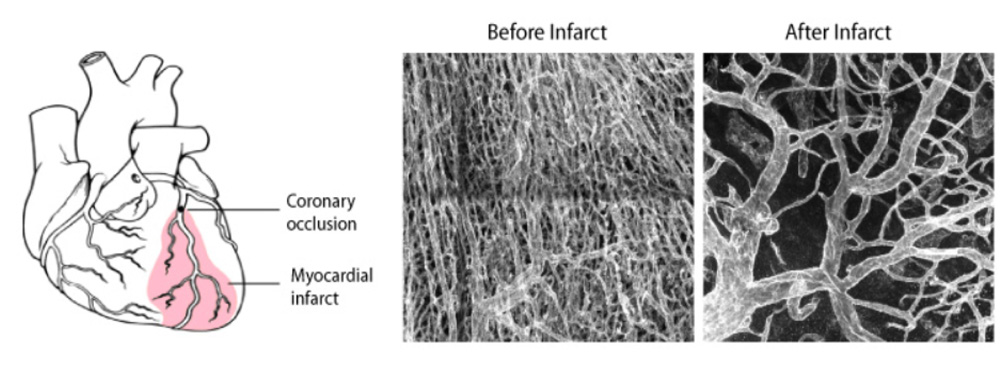
Figure 6 - Vascular remodelling after myocardial infarction.
Understanding gene function is pivotal for the progress of biomedical research. Traditional biomedical genetic studies compare cells from distinct mutant and control animals, a method that often fails to account for the differing epigenetic landscapes and tissue microenvironments within each animal. This disparity can lead to confusing results, complicating the interpretation of gene function in individual cells. With this in mind, our group developed recently new mouse models, imaging methods and single cell omics approaches to induce and analyse genetic mosaics with higher throughput (Figure 7 from Garcia-Gonzalez et al., 2024 Nature Methods). iFlpMosaics is a compendium of new mouse lines and related imaging and analysis methods that allows researchers to induce and analyse genetic mosaics with higher throughput and reliability, making it easier to study single cell-autonomous gene function. These methods improve our understanding of the effect of somatic genetic mutations during tissue development and in disease processes, such as mutations causing cancer or cardiovascular malformations.
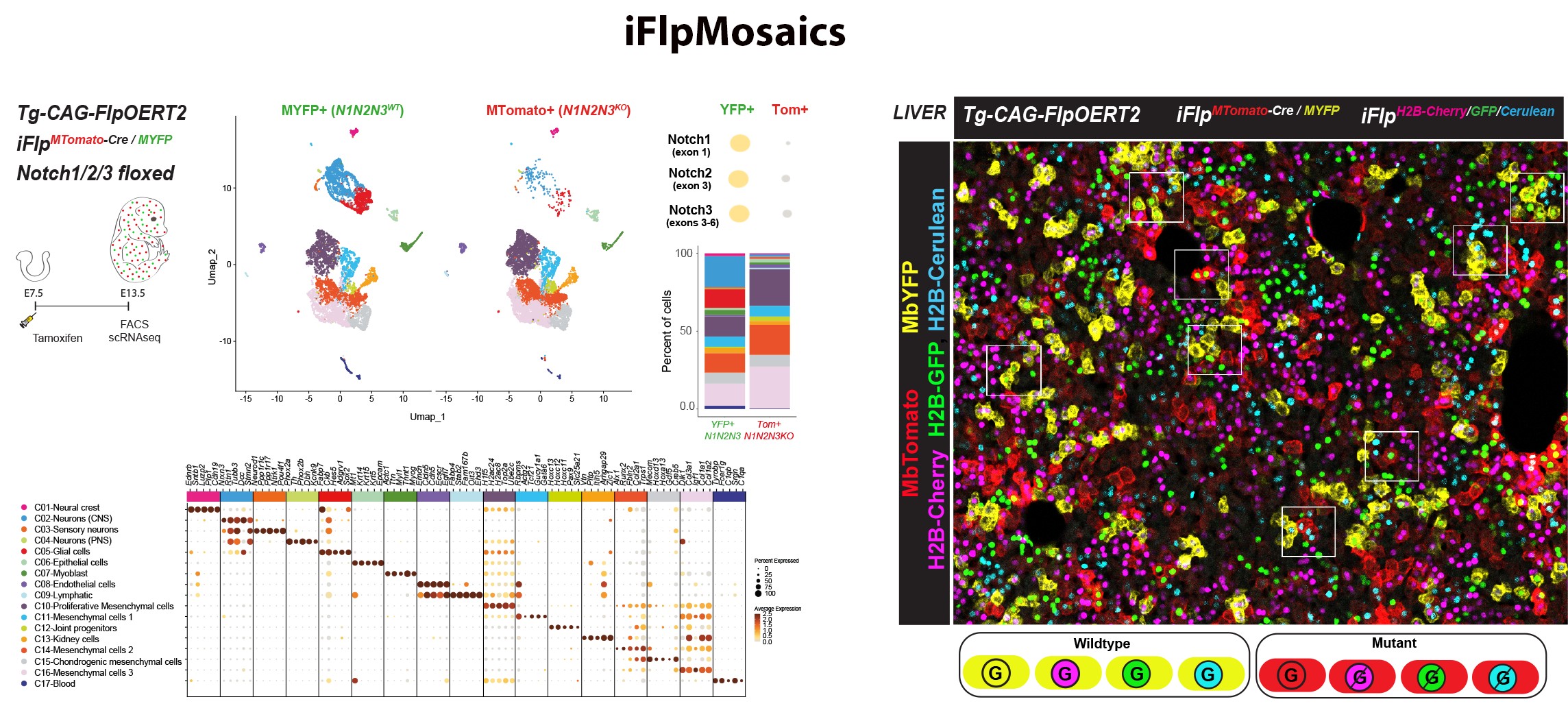
Figure 7. iFlpMosaics enable the multispectral barcoding and high-throughput
imaging or omics analysis of mutant and wildtype cells.






