JCI: A new study reveals a key mechanism driving atherosclerosis in Hutchinson-Gilford Progeria Syndrome
A team of scientists from the CNIC and the CSIC has identified a key mechanism in the development of atherosclerosis in patients with the rare genetic disease Hutchinson-Gilford progeria syndrome
A team of researchers from theCentro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), the Centro de Investigaciones Biológicas Margarita Salas (CIB-CSIC), and the Instituto de Ciencias de Materiales de Madrid (ICMM-CSIC) has made a significant breakthrough in understanding the underlying causes of cardiovascular disease in patients with Hutchinson-Gilford progeria syndrome (HGPS), an ultra-rare genetic disorder that accelerates the aging process. The most serious consequence of HGPS is the early onset of cardiovascular disease, leading to premature death at an average age of 14.5 years.
The study was led by Dr. Vicente Andrés, leader of the Molecular and Genetic Cardiovascular Pathophysiology group at the CNIC and principal investigator in the Spanish cardiovascular research network (CIBERCV), and Dr. Ignacio Benedicto, leader of the Vascular Aging group at CIB-CSIC and a visiting scientist at the CNIC.
In the study, the researchers identify the activation of the YAP/TAZ pathway in endothelial cells as a major contributor to the development of atherosclerosis in HGPS. The discovery, published in The Journal of Clinical Investigation, sheds light on the vascular problems faced by HGPS patients and opens up potential new avenues for treatment.
HGPS is caused by a mutation in the LMNA gene that leads to the synthesis of a toxic protein called progerin. This mutant protein disrupts normal cell function and accelerates cell aging. Children with HGPS typically show signs of rapid aging in the first two years of life, and by the time they reach their early teens most patients develop severe atherosclerosis—a condition in which the arteries stiffen and narrow—leading to heart attack, stroke, or heart failure, the main causes of premature death in HGPS patients. Despite the severity of this disease, the precise mechanisms underlying the cardiovascular problems in HGPS patients have remained poorly understood.
The authors explored how endothelial cells—the cells that line blood vessels—are affected in HGPS. Using advanced single-cell RNA-sequencing technology, they analyzed gene expression in the multiple cell types present in the arterial wall in a mouse model of HGPS and in healthy control mice. This approach allowed the researchers to examine the behavior of individual endothelial cells in unprecedented detail.
Este hallazgo, publicado en The Journal of Clinical Investigation, ofrece nuevas perspectivas para tratar los problemas vasculares en esta enfermedad
The results show that endothelial cells in HGPS undergo significant changes in gene expression related to inflammation, immune-cell recruitment, and the stiffening of the surrounding extracellular matrix. One of the most striking findings was the activation of the YAP/TAZ signaling pathway, a critical regulator of how cells respond to mechanical forces such as blood flow and the stiffness of their environment. In HGPS mice, this pathway was found to be abnormally active in endothelial cells from the aorta, the main artery carrying blood from the heart to the rest of the body.
First author Dr. Ana Barettino explained that "Our findings suggest that the stiffening of the arterial wall and the changes in blood flow patterns in HGPS trigger the activation of the YAP/TAZ pathway in endothelial cells. This in turn promotes inflammation and the accumulation of immune cells in the arteries, which accelerates the development of atherosclerosis."
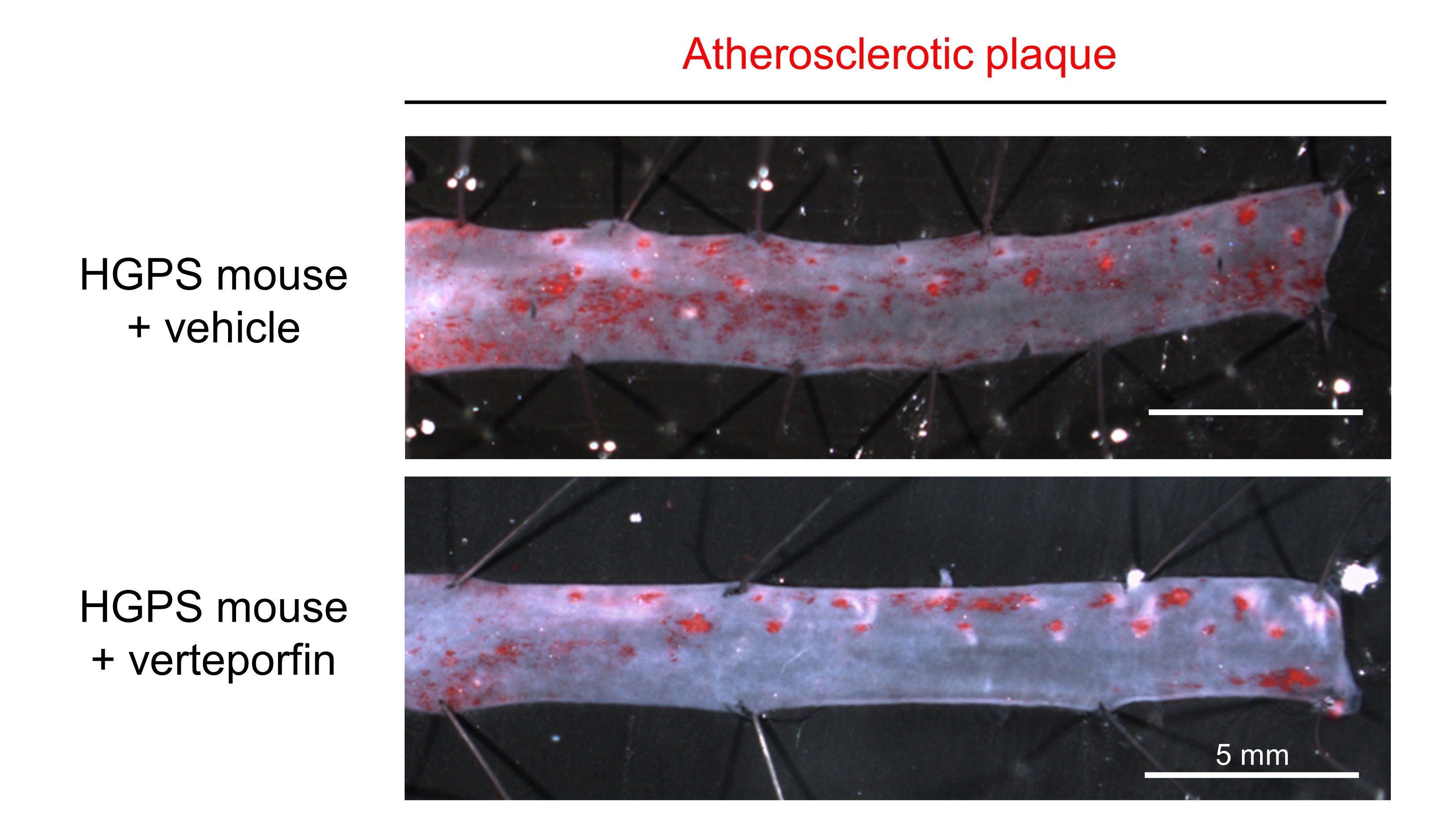
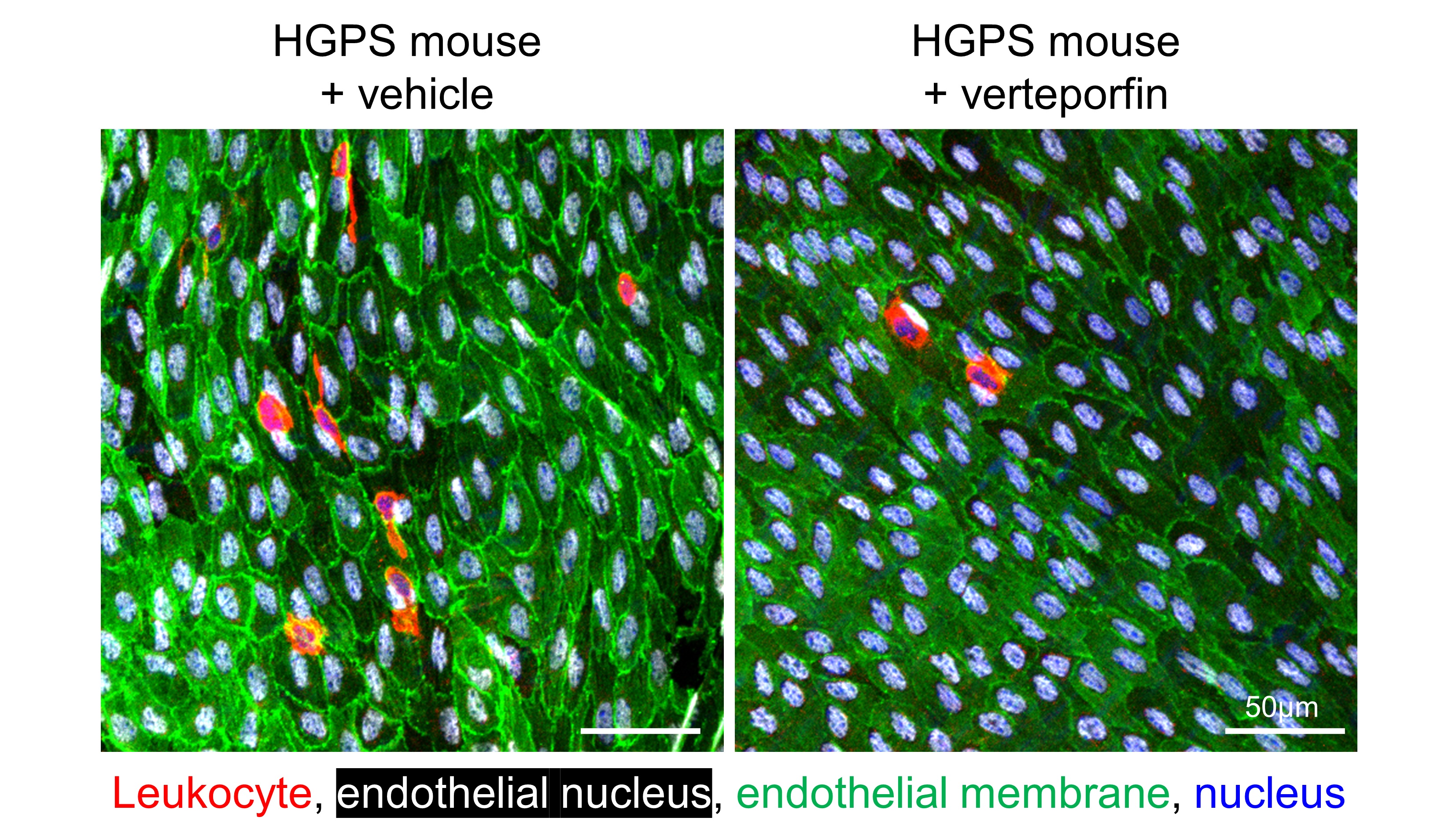
To explore the potential therapeutic implications of these findings, the team tested whether inhibiting the YAP/TAZ pathway could reduce the progression of atherosclerosis in HGPS mice. For this part of the study they used verteporfin, a drug already approved by the U.S. Food and Drug Administration and the European Medicines Agency for the treatment of age-related macular degeneration. When HGPS mice were treated with verteporfin, the researchers observed a significant decrease in atherosclerosis burden together with a marked reduction in both the activation of endothelial cells and the accumulation of immune cells in the aorta, two key contributors to atherosclerosis.
Dr. Vicente Andrés noted that "The results are very encouraging. By targeting the YAP/TAZ pathway, we were able to significantly reduce the progression of atherosclerosis in our HGPS mouse model. While more research is needed, this opens up the possibility of developing new therapies that could one day be used to treat not only HGPS but also other age-related cardiovascular diseases."
Although verteporfin showed promise in reducing atherosclerosis in HGPS mice, the researchers caution that more work is needed before the YAP/TAZ pathway can be safely targeted in patients. One concern is that the YAP/TAZ pathway is also involved in many normal biological processes, including tissue repair and regeneration. Therefore, any therapy aimed at inhibiting this pathway would need to be carefully adjusted to avoid unwanted side effects.
"Our study represents an important advance in understanding the mechanisms behind vascular aging in HGPS," said Dr. Barettino. "However, translating these findings into a safe and effective treatment for patients will require further research to determine how we can specifically target the YAP/TAZ pathway in diseased cells without affecting healthy tissue."
In addition to its significance for HGPS, the study has broader implications for understanding cardiovascular disease in the general population. Atherosclerosis is a leading cause of death worldwide, and many of the processes identified in this study—such as vascular stiffening and the activation of inflammatory pathways—also occur in the arteries of older adults.
"The insights we’ve gained from studying HGPS can help us gain a better understanding of the aging process in general and of the factors that contribute to cardiovascular disease in older individuals," added Dr. Andrés. Dr. Benedicto stressed that "By targeting the molecular pathways that drive vascular aging, we may be able to develop new therapies that extend healthy lifespan and improve quality of life."
The study was supported by grants from the Spanish Ministerio de Ciencia, Innovación y Universidades (MICIU) and Agencia Estatal de Investigación (AEI) (MICIU/AEI/10.13039/501100011033), ERDF/EU and “NextGenerationEU”/PRTR (PID2022-141211OB-I00, PID2022-137111OA-I00, RYC2021-033805-I), and theComunidad de Madrid with co-funding from the ESIF/EU (2017-T1/BMD-5247, 2021-5A/BMD-20944).











