Development Cell: A CNIC study reveals the key role of mitochondrial proteins in cardiac regeneration
Scientists at the CNIC, CIBER, and University of Berne identify the fundamental role of a family of proteins in an essential process for cellular energy production
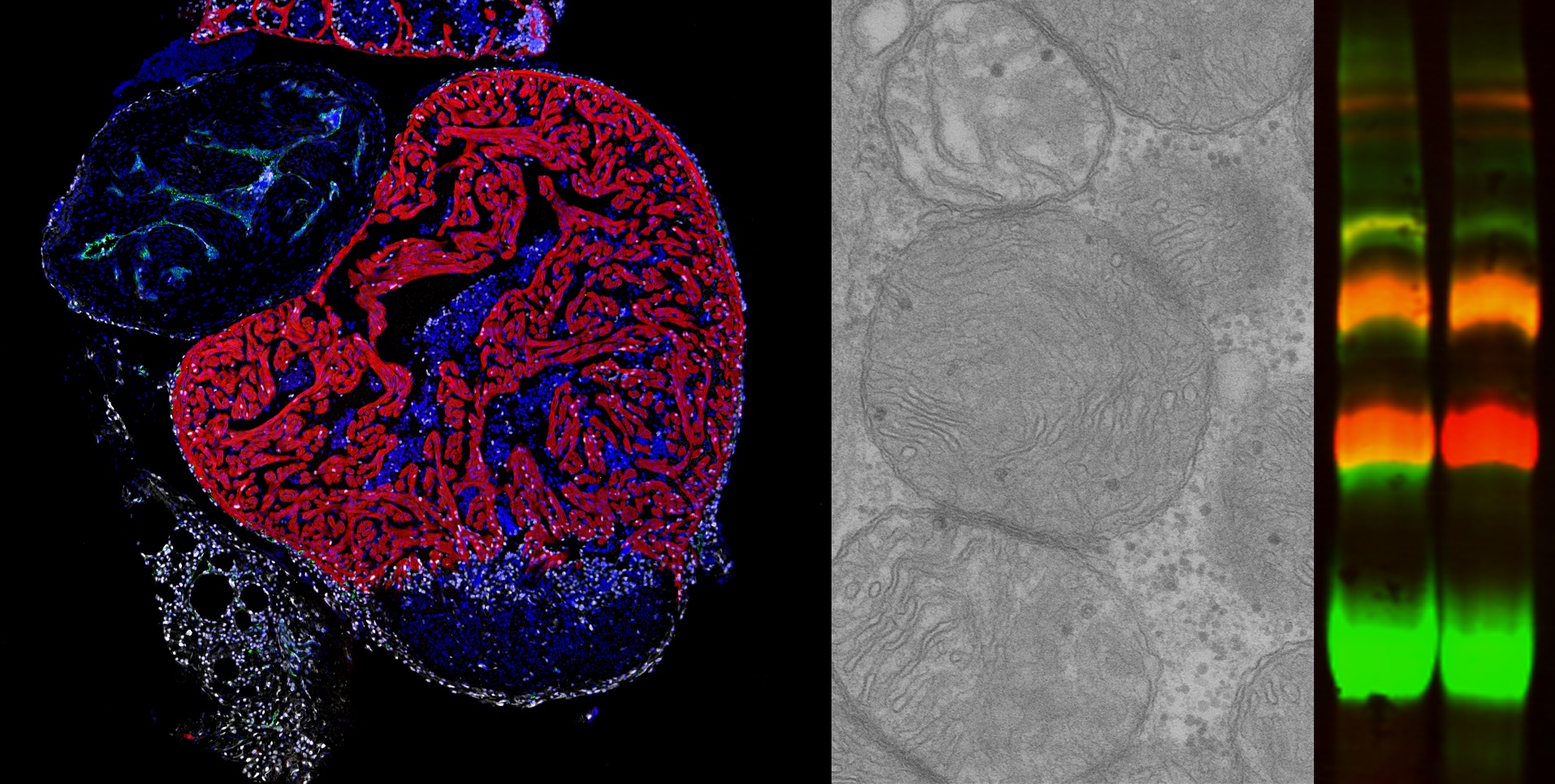
Mitochondria, known as the power plants of the cell, play an essential role in supplying the energy needed for correct cell function. Within mitochondria, energy production is generated by the respiratory chain, which is formed of five complexes called CI through CV. These complexes can assemble to form supercomplexes, but little is known about the role of this process or how it is controlled. Now, a new study explores the mechanisms of supercomplex assembly and uncovers a major impact of mitochondrial assembly factors on cardiac regeneration. The study was led jointly by Dr. José Antonio Enríquez at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) and Dra. Nadia Mercader, of the University of Berne in Switzerland, who is a visiting scientist at the CNIC.
The study, published in Development Cell, shows that a member of the Cox7a family of proteins plays a fundamental role in the assembly of CIV dimers and that this assembly is crucial for correct mitochondrial function, and thus for cellular energy production.
The Cox7a protein family includes three members: Cox7a1, Cox7a2, and Cox7a2l (also called SCAF1). Previous studies by both groups showed that when CIV contains SCAF1 it associates strongly with CIII to form a respiratory supercomplex known as the respirasome. In these previous studies, the authors postulated that inclusion of Cox7a2 would generate a CIV incapable of forming associations, while CIV molecules including Cox7a1 would associate together to form CIV homodimers. The new study demonstrates experimentally the role of Cox7a1 in the formation of these CIV homodimers.
Working with the zebrafish model, the researchers found that the absence of cox7a1 prevented the formation of CIV dimers, and the loss of these dimers influenced the weight and swimming ability of the affected fish. “Cox7a1 is mainly expressed in striated muscle cells, and it is precisely skeletal muscle tissue that was most affected by the lack of cox7a1 function. The other principal striated muscle type is the heart muscle, or myocardium,” explained Dr. Enríquez.
But while the loss of Cox7a1 in skeletal muscle was detrimental, its loss in cardiac muscle improved the regenerative response of the heart to injury. “This result shows that these proteins play a crucial role in the activation of the capacity for cardiac repair after injury,” explained study first author Carolina García-Poyatos.

To explore the function of Cox7a1 in more depth, CNIC investigators Enrique Calvo and Jesús Vázquez performed a proteomic study of the skeletal muscle and myocardium of zebrafish lacking Cox7a1, and this analysis was expanded by a metabolomic study conducted by colleagues at the University of Berne. This collaborative analysis revealed profound differences from unaltered fish with intact Cox7a1 expression. “These findings suggest that the molecules involved in mitochondrial supercomplex assembly can have a considerable effect on the control of metabolism, possibly opening the way to new treatments for cardiac diseases and other metabolic conditions,” said Dr. Mercader.
According to the research team, this discovery represents “a significant advance in the understanding of the cellular mechanisms involved in cardiac regeneration and could point the way to the development of therapies aimed at promoting cardiac regeneration.”
The authors conclude that mitochondrial assembly factors can significantly influence the control of metabolism.
The study was supported by the European Union Horizon 2020 programme (grants 874764 and 819717), the Human Frontier Science Program (grant RGP0016/2018), and the Swiss National Science Foundation (grant 320030E-164245).