EXCELLENCE IN THE SCIENCE COMMUNICATION
The main scientific journals científicas publish research from the CNIC laboratories
JACC: The sooner the better
Teaching healthy habits in elementary school reduces abdominal fat

A study by the CNIC and Fundación SHE, supported by “la Caixa” Foundation demonstrates that teaching healthy habits through classroom activities helps to prevent the accumulation of abdominal fat during the first school years. The study, published in the Journal of the American College of Cardiology (JACC), is one of the largest contemporary school-based health promotion studies and has one of the most extensive participant follow-up schedules.
The study included 1771 boys and girls attending 48 public elementary schools in the Comunidad de Madrid. The schools were divided into 4 groups. One group of 12 schools conducted a health-promotion intervention throughout the six years of elementary school; the intervention covered emotions management, acquisition of healthy eating habits, active living, and knowledge of the body and heart (SI! Program–Comprehensive Health). Another two groups conducted the same intervention but only for three years, one group during the first three years and the other group in the final three years. The fourth group did not conduct any specific health-related intervention.
Excess weight affects almost 1 of every 3 children in Spain, especially those in more vulnerable social groups. The results of this study suggest that interventions promoting healthy lifestyle habits can be more effective at reducing childhood obesity if implemented early, in the first years of elementary school.
JACC Clinical Electrophysiology
Genetics would explain a large number of cases of young adults with a pacemaker without an identified cause

A study led by CNIC cardiologists Juan Pablo Ochoa and Pablo García-Pavia, published in the JACC Clinical Electrophysiology, revealed that rare genetic variants increase the risks of cardiac conduction disorders in young adults who need to use a pacemaker. According to the study, 15% of cases have a direct genetic mutation, and an additional 30% have relevant genetic alterations. This underlines the importance of genetics in the diagnosis and management of these disorders.
Detecting genetic mutations not only allows for a more precise diagnosis and early treatment to prevent future complications, it also benefits the patient’s family members by identifying if they have inherited the mutation and are at risk.
The study analyzed 150 patients under the age of 60 who used pacemakers due to an unknown cause, comparing their genetic profile with that of a reference population. The results support the need to perform genetic tests in young patients with unexplained cardiac conduction disorders in order to improve treatment and family management. This is the biggest analysis about genetics and cardiac conduction disorders that has been done up until now.
Science Advances
Why physical exercise stimulates the desire to stay active: the molecular relationship between the muscle and the brain is revealed

A study led by Guadalupe Sabio, currently at the CNIO, with the participation of the CNIC, discovered a mechanism between the muscle and the brain that regulates the desire to do exercise. During exercise, the muscles activate the p38y protein, which increases the secretion of the interleukin 15 (Il-15) protein. This protein stimulates the brain’s motor cortex, which increases the motivation to exercise, according to data obtained in animal and human models.
The study shows that constant exercise boosts this effect, even in cases of obesity, improving metabolism and reducing the risks of diabetes and liver fat accumulation, without significant adverse effects. In humans, the levels of IL-15 increase with exercise, but they are lower in obese people, which could convert this into an interesting marker because of physical activity.
This discovery opens doors to designing more personalized workout programs and to possible treatments, such as a drug derived from the IL-15 protein for those who have more difficulty staying active, as well as for obese people. The next steps include researching the relationship between exercises, longevity and cancer, and exploring how different exercises affect this mechanism.
Nature Medicine
CNIC scientists discover a new cardiovascular risk factor and identify a drug able to reduce its effects

A new study published in Nature Medicine and carried out by researchers at the CNIC resolves this critical debate by establishing clonal hematopoiesis as a new risk factor for atherosclerosis—the formation of lesions in the arterial wall that underlies most cardiovascular disorders. In a second study, published in the European Heart Journal, the CNIC scientists propose the ancient medication colchicine as the central plank of personalized strategies to alleviate the effects of clonal hematopoiesis associated with acquired mutations in the TET2 gene. The results of these important studies will be presented today at the European Society of Cardiology meeting in London, UK.
Both studies highlight the potential of personalized strategies to prevent cardiovascular diseases by targeting these mutations.
The PESA study is cofunded by the CNIC and Santander Bank. The two studies were additionally funded by the Spanish Ministerio de Ciencia, Innovación e Universidades (PLEC2021-008194), the Spanish cardiovascular research network (CIBERCV), Fundación “la Caixa” (LCF/PR/HR17/52150007; LCF/PR/HR22/52420011), and Fundación ‘La Marató TV3’ (202314-31).
Circulation
A new mechanism of early-onset atherosclerosis in a premature aging syndrome

Scientists at CNIC have identified the process of endothelial-to-mesenchymal transition (EndMT) as a novel mechanism in premature atherosclerosis in progeria. The study, published in the journal Circulation, also proposes a new therapeutic target for this disease. The study was led by Dr. Vicente Andrés, leader of the Molecular and Genetic Cardiovascular Pathophysiology group at the CNIC and a principal investigator in the Spanish cardiovascular research network (CIBERCV), and Dr. Magda Hamczyk, a research fellow at the University of Oviedo and a CNIC visiting scientist. Atherosclerosis is marked by the accumulation of cells and cholesterol in arterial walls, leading to plaque formation that can obstruct arteries and cause life-threatening cardiovascular events. Research in this field is crucial for improving patient outcomes, as emphasized by Dr. Vicente Andrés.
Hutchinson-Gilford progeria syndrome (HGPS) is a rare genetic disorder characterized by premature aging and accelerated atherosclerosis, leading to early death from cardiovascular complications. Previous studies by Dr. Andrés and colleagues identified smooth muscle cell death as a key factor in this process.
The study explored how smooth muscle cell loss affects endothelial cells, leading to immune cell recruitment and increased LDL permeability, which accelerates plaque formation. The most significant discovery was the hyperactivation of endothelial-to-mesenchymal transition (EndMT), a process that exacerbates atherosclerosis.
The research also identified the hyperactivated TGFβ1–SMAD3 signaling pathway as a potential therapeutic target. Inhibiting this pathway with SIS3 alleviated vascular disease symptoms in progeroid mice. Dr. Andrés highlighted that studying rare diseases like progeria can contribute to treatments for broader conditions like atherosclerosis, a major global health concern.
The study was funded by the Ministerio de Ciencia, Innovación y Universidades (MICIU)/Agencia Estatal de Investigación (AEI) (/10.13039/501100011033) and ERDF/EU (PID2022-141211OB-I00). The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the MICIU, and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (grant CEX2020-001041-S funded by MICIN/AEI/10.13039/501100011033).
The RESILIENCE trial
Preventing heart injury caused by anticancer drugs
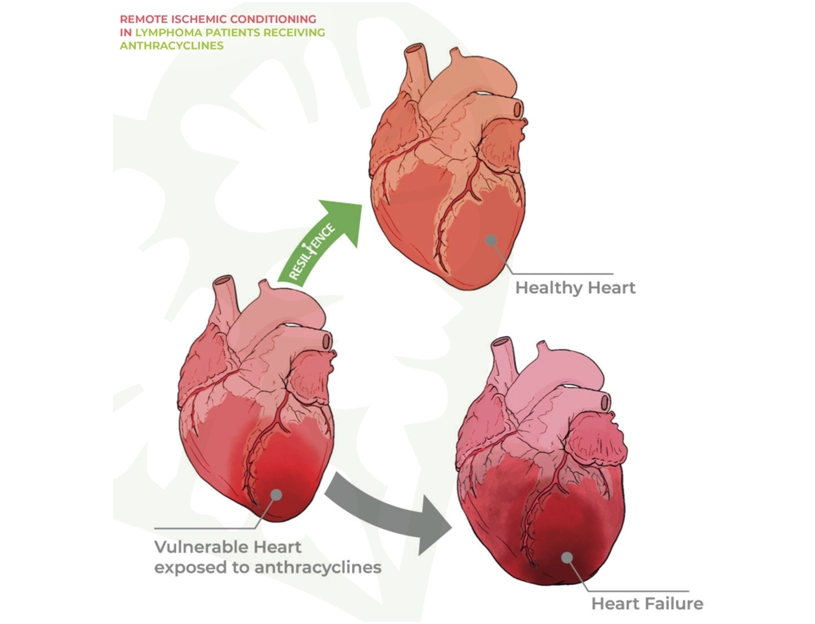
The RESILIENCE clinical trial has been designed to explore the effectiveness and safety of remote ischemic conditioning (RIC) used to prevent the cardiotoxic effects of anthracyline-based chemotherapy in patients with lymphoma. As described in a recent European Journal of Heart Failure editorial this international double-blind clinical trial promises to generate new knowledge and provide possible treatments for this major clinical challenge.
RESILIENCE is financed by the European Commission (H2020 Programme), and its goal is to reduce the prevalence of heart failure in cancer survivors and thus improve their quality of life. The trial is coordinated by the CNIC under the leadership of Dr. Borja Ibáñez, and among the key project partners is the European Society of Cardiology, which will lead one work package and participate in several other activities within the consortium.
Cancer patients are a vulnerable population at risk of developing cardiovascular complications. Some of these adverse cardiovascular effects are caused by drugs used to treat cancer. Anthracyclines are highly effective against many types of cancer, including the various forms of lymphoma, breast cancer, leukemia, melanoma, and uterine and gastric cancers. However, these drugs have a potential toxic effect on the heart that can lead to chronic heart failure.Another unique feature of the RESILIENCE trial is the use of the latest generation CMR technology to investigate the effect of RIC on heart function and composition. Study participants will be randomized to receive weekly RIC or a dummy procedure throughout their chemotherapy treatment. The patients will be examined by multiparametric CMR at three key time points: at the start of the study, halfway chemotherapy (i.e. after the third chemotherapy cycle, intermediate CMR), and two months after the end of the chemotherapy treatment. These CMR scans will monitor changes in heart function, with particular focus on left ventricular ejection fraction (LVEF).
The RESILIENCE trial represents a significant advance in the development of cardioprotective strategies for cancer patients with a high risk of anthracycline-induced cardiomyopathy. “All the patients included in the trial have some characteristic that places them at high risk of this cardiac complication secondary to cancer chemotherapy,” concluded Dr. Ibáñez.
The RESILIENCE trial is funded by the European Commission (H2020-HEALTH, grant number 945118).
Cell
Scientists at the CNIC discover an unexpected involvement of sodium transport in mitochondrial energy generation

The GENOXPHOS (Functional Genetics of the Oxidative Phosphorylation System) group at the CNIC has discovered a crucial role of sodium in the generation of cellular energy. The study, led by GENOXPHOS group leader Dr. José Antonio Enríquez, and published in the journal Cell, reveals that respiratory complex I, the first enzyme of the mitochondrial electron transport chain, possesses a hitherto unknown sodium transport activity that is crucial for efficient cellular energy production.
The discovery of this activity provides a molecular explanation for the origin of the neurodegenerative disease Leber’s hereditary optic neuropathy (LHON). First described in 1988, LHON is linked to defects in mitochondrial DNA and is the most frequent mitochondrially inherited disease in the world. The new study shows that the hereditary optic neuropathy in LHON is caused by a specific defect in the transport of sodium and protons by complex I.
Led by CNIC scientists José Antonio Enríquez and Pablo Hernansanz, the research team used an array of mutants and diverse genetic models to demonstrate that mitochondrial complex I exchanges sodium ions for protons, thus generating a gradient of sodium ions that parallels the proton gradient. This sodium gradient accounts for as much as half of the mitochondrial membrane potential and is essential for ATP production.
Discussing possible treatments for LHON, José Antonio Enríquez commented that while drugs are available that successfully replicate sodium transport across the inner membrane of isolated mitochondria, clinical use of these drugs is hindered by their toxic secondary effects on sodium transport in the cell membrane. “The challenge now is to design drugs that act specifically in mitochondria without effecting other parts of the cell,” said Dr. Enríquez.
The researchers also believe that defects in sodium–proton transport may play a role in other, more frequent neurodegenerative diseases such as Parkinson’s, in which an involvement of complex I has been detected.
The study was supported by the Ministerio de Ciencia e Innovación (MCIN) RTI2018-099357-B-I00, and CIBERFES (CB16/10/00282), the Human Frontier Science Program (grant RGP0016/2018), and Leducq Transatlantic Networks (17CVD04).
JACC Cardiovascular Imaging
Cardiometabolic risk factors in apparently healthy individuals are linked to altered coronary microcirculation
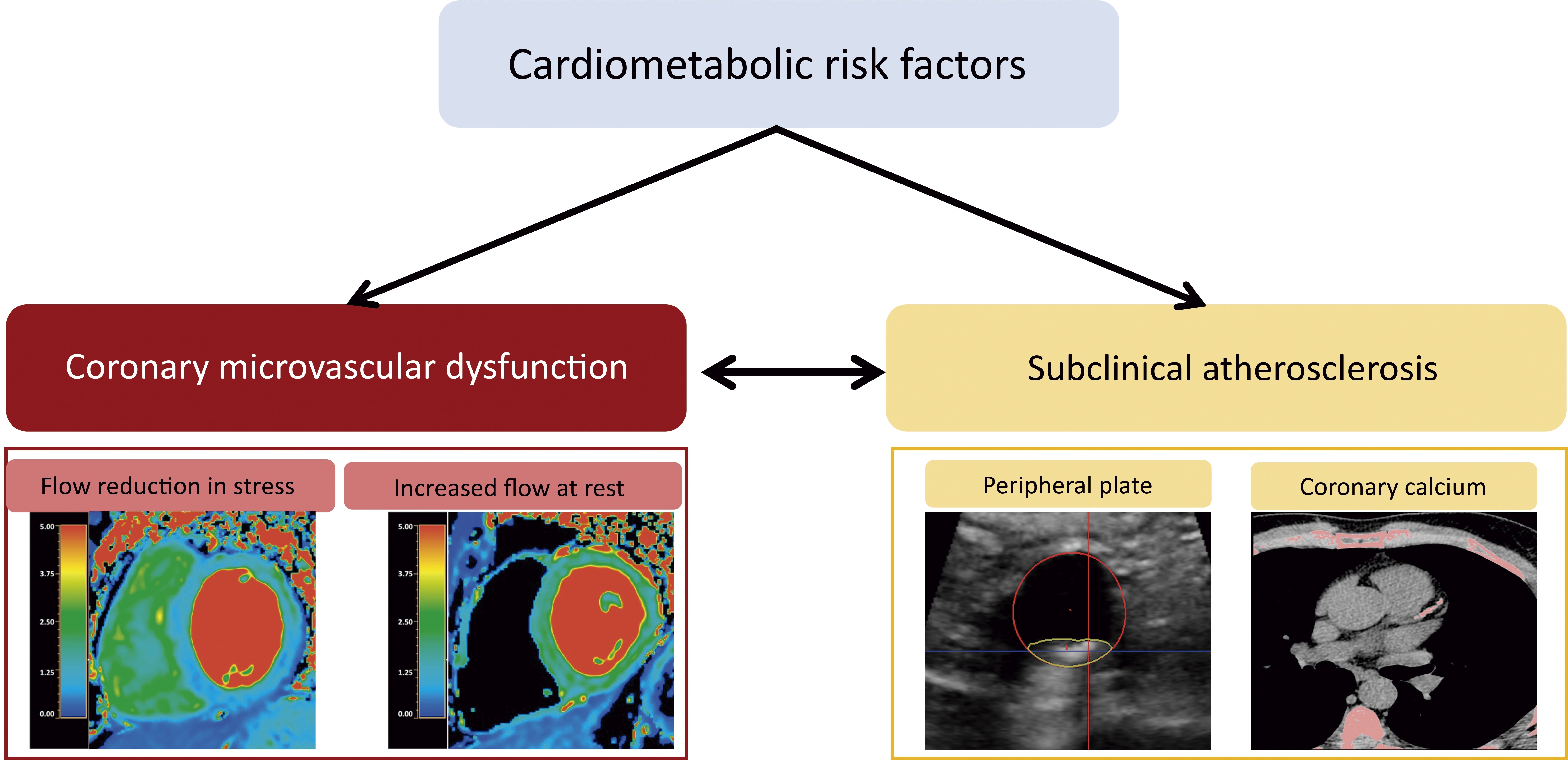
A study from CNIC reveals how risk factors and subclinical atherosclerosis affect heart microcirculation in asymptomatic middle-aged individuals. The research, published in JACC: Cardiovascular Imaging, highlights the importance of assessing the heart vessels’ ability to regulate blood flow and predict future cardiovascular risk. The study highlights the importance of assessing coronary microvascular function in individuals with no known cardiovascular disease in order to improve the prediction of atherosclerosis progression and future cardiovascular risk.
The results demonstrate that impaired coronary microvascular function is directly associated with the presence of cardiometabolic indicators such as metabolic syndrome, insulin resistance, and diabetes, as well as with subclinical atherosclerosis (the presence of fatty lesions in artery walls before the appearance of symptoms) in peripheral or coronary arteries.
Dr. Borja Ibáñez, who is the Scientific Director at the CNIC emphasized the value of examining PESA study participants: “The PESA population covers a crucial age group for the early detection of cardiovascular disease. By assessing microvascular function in this population, we were able to identify patterns that might have been hidden in other groups. Focusing on this age group gives us a unique window of opportunity for the implementation of primary prevention strategies, before the appearance of clinical symptoms.”
Over a three-year follow-up, atherosclerosis progression was less pronounced among participants who had better coronary microvascular function at the start of the study. This result underlines the potential of microvascular function as a key marker in the stratification of cardiovascular risk and the prevention of future events.
Overall, the study shows that, in a large cohort of asymptomatic middle-aged individuals with no ischemic heart disease, the presence of cardiometabolic risk factors and systemic atherosclerosis is associated with altered coronary microvascular function. The higher the burden of cardiometabolic risk factors and the greater the extent of subclinical atherosclerosis, the stronger the impairment of coronary microvascular function. Moreover, preserved coronary microvascular function is associated with a lower risk of atherosclerosis progression.
The PESA study is funded by the CNIC and Banco Santander. The study also received financial support from the European Commission (ERC-CoG 819775 and H2020-HEALTH 945118), The Spanish Ministerio de Ciencia e Innovación (PID2019-110369RB-I00), and the Red Madrileña de Nanomedicina en Imagen Molecular - Comunidad de Madrid (S2017/BMD-3867 RENIM-CM).
JACC
Progression of subclinical atherosclerosis predicts all-cause mortality risk

A study carried out at Mount Sinai Fuster Heart Hospital in New York in collaboration with the CNIC provides important new information about atherosclerosis, a disease in which lipids (cholesterol) and other substances accumulate in plaques on the arterial wall, causing the vessels to harden and narrow, and increasing the risk of severe cardiovascular conditions.
The study shows that both the burden of atherosclerosis and its progression (growth of plaques or the spread of the disease to new arteries) in asymptomatic individuals is independently associated with the risk of death from any cause.
The goal of the new study was to determine the independent predictive value of the burden and progression of subclinical atherosclerosis above and beyond prediction based on established cardiovascular risk factors.
The study included 5716 asymptomatic adults with an average age of 69 years (56.7% women) who were examined from 2008 to 2009 as part of the BioImage project, which examined a US population to evaluate factors implicated in atherosclerosis progression.
BioImage, led by Dr. Fuster, was the first study to demonstrate the value of 3D echocardiography and other advanced imaging technologies to detect atherosclerotic disease of the large vessels long before the appearance of symptoms. “The long asymptomatic phase of the disease presents a window of opportunity that has not been exploited in the younger population,” said Fuster, who is the lead author on the JACC study.
The study, concluded Dr. Fuster, demonstrates that detecting subclinical atherosclerosis early and monitoring its progression can improve the prediction and prevention of death from any cause, offering a valuable tool for clinical practice “Vascular ultrasound is a noninvasive and affordable test, and the valuable prognostic information it provides can be used to improve risk stratification and to target lifestyle recommendations for the control of cardiovascular risk factors”, underlined Dr. Borja Ibáñez, CNIC Scientific Director, a cardiologist at Fundación Jiménez Díaz, and a member of the Spanish cardiovascular research network (CIBERCV).
The study, concluded Dr. Fuster, demonstrates that detecting subclinical atherosclerosis early and monitoring its progression can improve the prediction and prevention of death from any cause, offering a valuable tool for clinical practice.
EUROPACE
A new image processing strategy for cardiac magnetic resonance

The, methodology validated in a multicenter study led by the Hospital Clínico San Carlos and the CNIC, enables preoperative planning based on a cardiac magnetic resonance imaging strategy that avoids the biases intrinsic to conventional image analysis.
The study has described and validated a new strategy for guiding ablation procedures in patients with complex tachycardias. Ablation procedures use energy—usually heat or cold—to eliminate small areas of heart tissue that cause pathological cardiac arrhythmias, thereby restoring normal heart rhythm. This type of procedure is frequently used to treat ventricular tachycardias originating in areas affected by scarring after a myocardial infarction.
The new approach uses advanced methods for processing cardiac magnetic resonance (CMR) images to identify the areas that maintain ventricular tachycardia in heart regions affected by postinfarction scarring. The method allows images to be processed systematically, avoiding the biases that can arise when CMR imaging parameters are selected manually. This systematic image processing increases the sensitivity for the detection of the regions responsible for these types of arrhythmias; moreover, the new strategy supports preoperative planning by allowing operators to accurately detect these regions before starting the ablation procedure.
The study used a swine model of myocardial infarction to analyze how variability in image processing parameters impedes accurate detection of the cardiac tissue circuits that maintain complex ventricular tachycardias. Using this animal model, the researchers designed a strategy that avoids this problem, and the method was later validated in patients in a multicenter study conducted between 2013 and 2022 and involving leading national and international experts.
The simplified planning of ablation procedures with the new method is especially useful in patients for whom conventional invasive catheter mapping is contraindicated due to the risk of inducing more severe tachycardias and circulatory collapse. In place of these risky procedures, the new method uses cardiac images obtained before the ablation procedure to identify target areas without the need to induce tachycardia during the procedure, reducing risk without reducing efficacy.
The study helps to avoid gaps in image integration when planning the ablation of complex ventricular tachycardias and is especially useful in patients with poorly tolerated episodes.
The new strategy is highly applicable in the clinic and can be easily implemented in commercial systems currently used to guide the ablation of postinfarction ventricular tachycardias.
The study was supported by the Spanish Ministerio de Ciencia e Innovación (MCIN) and the Pro-CNIC Foundation in relation to the CNIC’s status as a Severo Ochoa Center of Excellence (CEX2020-001041-S). The study was also supported by grants from the MCIN (PID2019-109329RB-I00), the Asociación de Ritmo Cardiac of the Spanish Society of Cardiology, the Fundación Interhospitalaria para la Investigación Cardiovascular, and the Fundación Fundación Eugenio Rodríguez Pascual.
JCI
A new study reveals a key mechanism driving atherosclerosis in Hutchinson-Gilford Progeria Syndrome

A team of scientists from the CNIC and the CSIC has identified a key mechanism in the development of atherosclerosis in patients with the rare genetic disease Hutchinson-Gilford progeria syndrome. The researchers has made a significant breakthrough in understanding the underlying causes of cardiovascular disease in patients with Hutchinson-Gilford progeria syndrome (HGPS), an ultra-rare genetic disorder that accelerates the aging process. The most serious consequence of HGPS is the early onset of cardiovascular disease, leading to premature death at an average age of 14.5 years.
In the study, the researchers identify the activation of the YAP/TAZ pathway in endothelial cells as a major contributor to the development of atherosclerosis in HGPS. The discovery, published in The Journal of Clinical Investigation, sheds light on the vascular problems faced by HGPS patients and opens up potential new avenues for treatment.
HGPS is caused by a mutation in the LMNA gene that leads to the synthesis of a toxic protein called progerin. This mutant protein disrupts normal cell function and accelerates cell aging. Children with HGPS typically show signs of rapid aging in the first two years of life, and by the time they reach their early teens most patients develop severe atherosclerosis—a condition in which the arteries stiffen and narrow—leading to heart attack, stroke, or heart failure, the main causes of premature death in HGPS patients. Despite the severity of this disease, the precise mechanisms underlying the cardiovascular problems in HGPS patients have remained poorly understood.
The authors explored how endothelial cells—the cells that line blood vessels—are affected in HGPS. Using advanced single-cell RNA-sequencing technology, they analyzed gene expression in the multiple cell types present in the arterial wall in a mouse model of HGPS and in healthy control mice. This approach allowed the researchers to examine the behavior of individual endothelial cells in unprecedented detail.
In addition to its significance for HGPS, the study has broader implications for understanding cardiovascular disease in the general population. Atherosclerosis is a leading cause of death worldwide, and many of the processes identified in this study—such as vascular stiffening and the activation of inflammatory pathways—also occur in the arteries of older adults.
The insights we’ve gained from studying HGPS can help us gain a better understanding of the aging process in general and of the factors that contribute to cardiovascular disease in older individual. By targeting the molecular pathways that drive vascular aging, we may be able to develop new therapies that extend healthy lifespan and improve quality of life.
The study was supported by grants from the Spanish Ministerio de Ciencia, Innovación y Universidades (MICIU) and Agencia Estatal de Investigación (AEI) (MICIU/AEI/10.13039/501100011033), ERDF/EU and “NextGenerationEU”/PRTR (PID2022-141211OB-I00, PID2022-137111OA-I00, RYC2021-033805-I), and the Comunidad de Madrid with co-funding from the ESIF/EU (2017-T1/BMD-5247, 2021-5A/BMD-20944).
Nature Communications
A key mechanism in the development and functioning of the conduction cardiac system is discovered
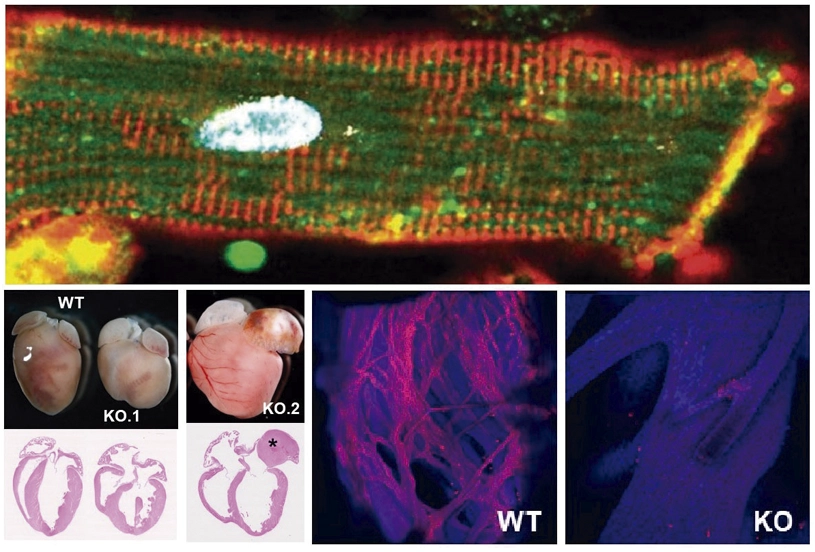
A study published in by a research team from the Carlos III Rare Diseases Research Institute (ISCIII), CIBER, CNIC Pompeu Fabra University (UPF) and Severo Ochoa Center for Molecular Biology (CBM, CSIC-UAM) has identified the Dhx36 protein as an essential regulator for the development and functioning of the heart, particularly in the conduction cardiac system.
This breakthrough, led by scientists Pablo Gómez del Arco (ISCIII), Pura Muñoz-Cánoves (UPF and Altos Labs), and Juan Miguel Redondo (CBM, CSIC-UAM), has shown that Dhx36 modulates gene networks that control the cardiomyocyte differentiation, through the resolution of G-quadruplex structures in the promoters of key genes of the cardiac conduction system. This function is crucial for the formation of specialized cells that make up the conduction system that transmits and controls the heart’s electric impulses.
The study also demonstrated that the absence of Dhx36 in the mouse cardiomyocytes during embryonic development and in adulthood provokes severe cardiac problems, including dilated cardiomyopathy and blockage in the transmission of the electrical impulse between the chambers of the heart, particularly between the atria and ventricles. These findings suggest that Dhx36 is essential for maintaining heart health, especially after birth, and that defects in this protein could be related to heart diseases that affect the electrical activity of the heart and cause dilated cardiomyopathy.
The study also identified the genes and signaling pathways involved in cardiac cell differentiation and in the development of the specialized fiber system of the Purkinje system, essential for the synchronization of ventricular contraction. The results suggest that transcriptional regulation plays a crucial role in cardiac function and opens new perspectives for the development of therapies to treat disorders in the cardiac conduction system, like those associated with heart failure.
Science Advances
A new CNIC study describes a mechanism whereby cells respond to mechanical signals from their surroundings

A study conducted at the CNIC, led by Dr. Jorge Alegre-Cebollada, has revealed the fundamental role of tissue viscoelasticity—a property still largely unexplored—in cellular function. The extracellular matrix (ECM), a network of proteins that supports and connects cells, influences processes such as cell migration, proliferation, and differentiation through its mechanical properties, including stiffness and viscoelasticity.
Until now, research on the mechanical properties of the ECM has primarily focused on stiffness, especially in the context of diseases like myocardial infarction and certain types of cancer. However, the cellular response to viscoelasticity has not been fully understood, particularly in stiffer tissues. The study, published in Science Advances, demonstrates for the first time how tissue viscoelasticity plays a crucial role in the process known as cellular homeostasis, which is the ability of cells to maintain an adequate internal balance for proper function.
According to Dr. Carla Huerta-López, who led the study, viscoelasticity regulates the time cells need to respond to mechanical stimuli. Using the example of a viscoelastic mattress that takes time to return to its shape after being pressed, Huerta-López compares this process to how cells require time to recover from mechanical alterations, such as a handshake or a blow.
The research team developed protein-based biomaterials that mimic the mechanical properties of the ECM to study how cells respond to viscoelasticity. Using these biomaterials and a computational model, the researchers discovered a mechanism in which tissue viscoelasticity counteracts the cellular response to stiffness in an unexpected way. This finding contradicts previous models and provides new explanations about how cells react to the mechanical properties of their environment, which could have implications for improving artificial tissues and treating diseases associated with changes in tissue viscoelasticity, such as certain types of cancer and cardiovascular diseases.
The study was made possible thanks to funding from the Ministry of Science, Innovation and Universities, the European Research Council (ERC), and the Community of Madrid through the interdisciplinary consortium Tec4Bio-CM. Notably, four principal investigators from Tec4Bio-CM directly contributed to this work from CNIC, ICMM-CSIC, and the Polytechnic University of Madrid.
Nature Communications
CNIC scientists discover a key mechanism in fat cells that protects the body against energetic excess

A team at the (CNIC) led by Professor Miguel Ángel del Pozo Barriuso, who heads the Mechanoadaptation and Caveolae Biology group at the CNIC, has identified an essential mechanism in fat cells (adipocytes) that enables them to enlarge safely to store energy. This process avoids tissue damage and protects the body from the toxic effects of accumulating fat molecules (lipids) in inappropriate places. The results, published in Nature Communications, signify a major advance in the understanding of metabolic diseases. Moreover, this discovery opens the door to the development of new therapeutic strategies to combat diseases related to chronic energetic excess, such as overweight, obesity, lipodystrophy, and metabolic syndrome, and their grave cardiovascular and metabolic complications. The team analyzed the role of caveolae, small invaginations in the cell membrane that act as sensors and shock absorbers of these stresses. “When an adipocyte accumulates fat and its surface is under increased tensile stress, the caveolae flatten, releasing a ‘reservoir’ of membrane that allows the cell to enlarge without breaking apart. Conversely, when fat reserves diminish, these structures regroup to reduce the excess membrane and restore cellular stability,” explained study first author Dr. María Aboy Pardal. The CNIC study highlights the key role of the caveolae protein caveolin-1 (Cav-1). For caveolae to flatten correctly in response to fluctuations in mechanical tension in the cell membrane, Cav-1 needs to be chemically altered by the addition of a phosphoryl group to a specific amino acid, a process called phosphorylation. For the study, the researchers developed a transgenic mouse that expresses a genetically altered version of Cav-1 that cannot be phosphorylated.
The study was funded by the Ministerio de Ciencia, Innovación y Universidades (MICIU) through the Agencia Estatal de Investigación (AEI) and with funding from the European Regional Development Fund “A way to make Europe” (SAF2017-83130-R, IGP-SO grant MINSEV1512-07-2016, BFU2016-81912-REDC, and SAF2020 (PID2020-118658RB-I00)) and by the Fundación “la Caixa” (AtheroConvergence, HR20-00075), the Community of Madrid regional government (Tec4Bio-CM, S2018/...), Fundació La Marató de TV3 (201936-30-31), and the Asociación Española Contra el Cáncer (PROYE20089DELP).
Nature Methods
CNIC presents iFlpMosaics, an innovative genetic toolkit for the study of gene function

A team at the CNIC, led by Dr. Rui Benedito, has developed a comprehensive set of innovative genetic tools and mouse lines, called iFlpMosaics, designed to enhance the study of gene function and its implications in health and disease.
The groundbreaking study, published in Nature Methods, presents a pioneering approach that overcomes critical limitations of existing methods for generating genetic mosaics. These innovations will enable scientists to more accurately investigate the effects of somatic mutations on cellular biology and disease.
The study highlights the iFlpMosaics toolkit’s utility across different experimental setups, detailing how it allows scientists to track the effects of single or multiple gene deletions within the same tissue. This advance opens the way to deeper insight into the function of genes in cell biology, regeneration, and disease.
The iFlpMosaics toolkit is unburdened by these shortcomings and allows researchers to induce genetic mosaics with high throughput and precision, making it easier to study cell-autonomous gene function directly within the same organism.
The toolkit not only enhances the understanding of genetic mutations in tissue development and disease processes, but also facilitates the study of complex interactions between cells within their microenvironment.
“iFlpMosaics offers a big step forward for researchers studying diseases caused by somatic mutations, such as cancer and vascular malformations” said Dr. Rui Benedito. “It’s precision and versatility provide an important resource for anyone seeking a better understanding of gene function in normal organ development and function, as well as in disease settings.”
The study was funded by the European Research Council (ERC) through Starting Grant AngioGenesHD (638028) and Consolidator Grant AngioUnrestUHD (101001814), the Spanish Ministry of Science, Innovation, and Universities (SAF2017-89299-P y PID2020-120252RB-I00), and the “la Caixa” Foundation (HR19-00120 and HR22-00316 AngioHeart).













