Excellence in communication science
Leading journals publish CNIC science
Nature Cardiovascular Research
The ‘guardian of the genome’ protects against cardiovascular disease

A team at the CNIC, working in collaboration with institutes in the USA, has demonstrated that acquired mutations in the gene encoding the protein p53 contribute to the development of atherosclerotic cardiovascular disease. Known as the Guardian of the Genome, p53 helps to maintain the integrity of the hereditary material inside cells by regulating multiple cell functions in response to cellular stresses.
Every day, an adult person generates hundreds of thousands of blood cells. Though essential, this process unavoidably promotes the appearance of mutations in the progenitor cells responsible for this production.
The presence of acquired p53 gene mutations in blood cells increases the risk of developing various types of cancer, including blood cancers.
In the new study, published in Nature Cardiovascular Research, the CNIC group led by José Javier Fuster demonstrates that p53 mutations also accelerate the development of atherosclerosis, the underlying cause of most cardiovascular disease, which is the leading cause of death in the world and places an enormous burden on health care systems.
The scientists analyzed sequencing data from the blood cells of more than 50,000 people.
“We found that carriers of acquired mutations in p53 had a higher risk of developing coronary heart disease and peripheral artery disease, and this effect was independent of established cardiovascular risk factors like hypertension or elevated blood cholesterol,” explained Dr. José Javier Fuster.
Based on these results, the CNIC scientists conducted functional studies in animal models of atherosclerosis, into which they introduced cells carrying p53 mutations.
The results showed that mice carrying these mutations developed cardiovascular disease more rapidly, mostly due to an abnormally elevated proliferation rate of immune cells in the artery walls.
“This combination of observations in humans with experimental studies in animals provides solid evidence that these mutations increase the risk of developing cardiovascular disease,” said Dr. Fuster.
For Dr. Valentín Fuster, CNIC General Director and an author on the study, these findings “broaden our knowledge of how the acquisition of mutations in blood cells, a phenomenon called clonal hematopoiesis, acts as a cardiovascular risk factor.”
The researchers highlight that mutations in different genes contribute to cardiovascular disease through distinct mechanisms. “In the future, this could be exploited to design personalized prevention strategies targeting the specific effects of different mutations,” said CNIC scientist Nuria Matesanz, one of the co-first authors on the study.
The CNIC study team received support from Fundación “la Caixa”, the Leducq Foundation, a Fundación BBVA 2019 Leonardo grant for researchers and cultural creators, and the Instituto de Salud Carlos III (ERA-CVD ‘CHEMICAL’ consortium).
The study was also supported by the US National Institutes of Health and the Department of Veterans Affairs.
Immunity
CNIC scientists identify a new therapeutic target in macrophages for the treatment of obesity-related diseases

A team at the CNIC has discovered that the metabolic requirements of macrophages differ depending on the organ in which they reside. In other words, these cells adapt to the needs of the organ in which they are located. The discovery “gives us a better understanding of how macrophages regulate their metabolism according to the organ in which they reside. In addition, our results reveal a vulnerability of macrophages that contributes to chronic inflammatory diseases and that could be exploited therapeutically for the treatment of conditions associated with obesity and metabolic syndrome, such as cardiovascular disease,” said study leader Dr. David Sancho, who heads the CNIC Immunobiology group. The study was published in an article in the journal Immunity.
Macrophages are immune cells that are normally distributed throughout the body and help to cleanse organs of all types of biological material that needs to be removed, from harmful particles such as mineral crystals or viruses to proteins or larger complexes that arise during development. Macrophages are also important for removing dead cells, thus contributing to tissue renewal.
The new study reveals that macrophages adapt their metabolism and function to the organ in which they reside. “In tissues with abundant extracellular fat and cholesterol, such as the lungs and spleen, macrophages adapt their metabolism to degrade these fats through mitochondrial respiration,” explained first author Dr. Stefanie Wculek. “Using genetic or pharmacological methods to disrupt mitochondrial respiration, mitochondria can be eliminated from lung and spleen, whereas the macrophages in other organs, which don’t depend on mitochondrial respiration, survive.”
Another example is provided by the macrophages located in body fat, or adipose tissue. “Macrophages residing in the body fat of a person of normal weight are unaffected by mitochondria-disrupting treatments because their metabolism is less dependent on mitochondrial respiration. This is because the fat cells, called adipocytes, are fully functional, leaving the macrophages in a resting state,” said Dr. Sancho. “However, in obese individuals, the excess fat surpasses the capacity of the adipocytes, and the resident macrophages become activated, converting into inflammatory cells that promote the development of insulin resistance, type 2 diabetes, and fatty liver.”
But this change in adipose tissue macrophages also makes them vulnerable.
The investigators conclude that this finding opens the way to new treatments for conditions linked to obesity and metabolic syndrome, like cardiovascular disease.
The studio was supported with grants from the International Human Frontier Science Program Organization; Fundación “la Caixa”; the Spanish Ministerio de Ciencia e Innovación (MCIN); the Agencia Estatal de Investigación (AEI) with the European Regional Development Fund (EDRF); the Spanish biomedical research network on frailty and healthy aging (CIBERFES-ISCiii); an ERC-2016-Consolidator Grant from the European Union Horizon 2020 Programme; and the Community of Madrid regional government (Inmunothercan-CM).
eClinicalMedicine
Imaging the adolescent heart
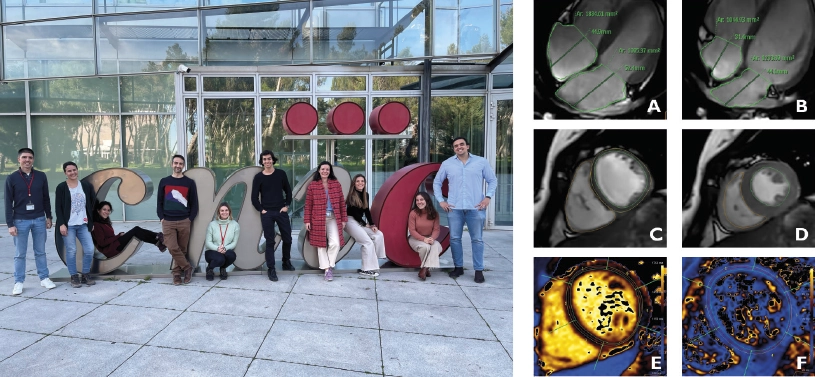
Magnetic resonance imaging (MRI) has allowed scientists at the CNIC to produce an accurate picture of the healthy heart in adolescence. Using this advanced technology, the research team was able to determine reference values for anatomical and functional parameters in the heart during adolescence. This information, pubished in eClinicalMedicine, has direct implications for clinical practice.
“Magnetic resonance imaging has become a very important method for studying the heart because it avoids exposing patients to radiation and provides more information, and with greater precision, than ultrasound, currently the most frequently used cardiac imaging technique,” said CNIC General Director Dr. Valentín Fuster, a co-author on the study.
Nevertheless, most published MRI data from adolescent subjects come from patients with congenital heart defects or other heart conditions. As a result, there is a lack of knowledge about the ‘normal’ values of cardiac parameters in the general adolescent population. “These reference values are essential for a proper interpretation of cardiac MRI studies in this population group,” said lead study author Dr. Rodrigo Fernández-Jiménez, leader of the Cardiovascular Health and Imaging group at the CNIC and a cardiologist at Hospital Clínico San Carlos.
These reference values are precisely what the CNIC team set out to define. As part of the EnIGMA project (Early ImaginG Markers of unhealthy lifestyles in Adolescents), the team managed, in the middle of the Covid-19 pandemic, to recruit 123 adolescent participants (64 girls and 59 boys) from seven public-funded secondary schools within the Comunidad de Madrid. The schools and adolescents were already signed up to the SI! Program for Secondary Schools, a program dedicated to promoting healthy lifestyle habits that is coordinated by Fundación SHE-“la Caixa” in partnership with the CNIC and the University of Barcelona.
“The response of the participants and their families was incredible,” said first author Dr. Carlos Real, a CNIC investigator and a resident cardiologist at Hospital Clínico San Carlos. “Some of the schools are located more than 60 km from the city, and the participants and at least one parent or guardian visited the CNIC’s advanced imaging facility on an entirely voluntary basis. Without their willingness to participate the project would not have been possible.”
Dr. Borja Ibáñez, CNIC Scientific Director and a co-author on the study, stressed that “the results have direct implications for clinical practice because they providde a list of reference values for multiple cardiac parameters used in daily practice, including measures of the size and functioning of the heart chambers (atria and ventricles) and cardiac tissue composition.” Dr. Fernández-Jiménez concluded that “with this information, physicians at any center can determine if cardiac MRI data from an adolescent’s heart fall within the normal range for this age group, and prescribe closer follow-up and additional tests if needed.”
JACC
High-dose anticoagulation can reduce intubations and improve survival for hospitalized Covid-19 patients

High-dose anticoagulation can reduce deaths by 30 percent and intubations by 25 percent in hospitalized Covid-19 patients who are not critically ill when compared to the standard treatment, which is low-dose anticoagulation. These are the significant findings from the large-scale international “FREEDOM” trial, led by Valentín Fuster, General Director of CNIC, President of Mount Sinai Heart and Physician-in-Chief of the Mount Sinai Hospital.
The study results were announced at the American College of Cardiology Scientific Sessions, together with World Congress of Cardiology in New Orleans (USA), and simultaneously published in the Journal of the American College of Cardiology (JACC).
“What we learned from this trial is that many patients hospitalized with Covid-19 with pulmonary involvement, but not yet in the intensive care unit (ICU), will benefit from high-dose subcutaneous enoxaparin or oral apixaban to inhibit thrombosis and the progression of the disease,” says Dr. Fuster. “This is the first study to show that high-dose anticoagulation may improve survival in this patient population—a major finding since Covid-19 deaths are still prevalent.”
This work was prompted by the discovery early in the pandemic that many patients hospitalized with Covid-19 developed high levels of life-threatening blood clots. Mount Sinai research showed that treatment with prophylactic (low-dose) anticoagulation was associated with improved outcomes both in and out of the intensive care unit among hospitalized Covid-19 patients. Researchers further observed that therapeutic (high-dose) anticoagulation might lead to better results. Then, they designed the FREEDOM Covid Anticoagulation Strategy Randomized Trial to look further into the most effective regimen and dosage for improving outcomes of hospitalized Covid-19 patients who are not critically ill.
Researchers enrolled 3,398 hospitalized adult patients with confirmed Covid-19 (median age 53) from 76 urban and rural hospitals across 10 countries —including hospitals within the Mount Sinai Health System in New York City— between August 26, 2020, and September 19, 2022. Patients were not in the ICU or intubated, and approximately half of them had signs of COVID-19 impacting their lungs with acute respiratory distress syndrome (ARDS). Patients were randomized to receive doses of three different types of anticoagulants within 24-48 hours of being admitted to the hospital and followed for 30 days. Equal numbers of patients were treated with one of three different drug regimens: prophylactic subcutaneous enoxaparin, therapeutic subcutaneous enoxaparin, and therapeutic oral apixaban. They compared the combined therapeutic groups to the prophylactic group.
The primary endpoint was a combination of death, requirement for ICU care, systemic thromboembolism, or ischemic stroke at 30 days. This endpoint was not significantly reduced between the groups. However, 30-day mortality was lower for those treated with therapeutic anticoagulation (high dose) compared with those on the prophylactic regimen (low dose). Seven percent of patients treated with the prophylactic anticoagulation died within 30 days compared with 4.9 percent of patients treated with therapeutic anticoagulation—an overall reduction of 30 percent. The need for intubations was also reduced in the therapeutic group: 6.4 percent of patients on the therapeutic regimen were intubated within 30 days compared with 8.4 percent in the prophylactic group—a 25 percent reduction.
The study showed therapeutic anticoagulation to be especially beneficial for patients with ARDS, a condition where Covid-19 damages the lungs. Among patients with ARDS at the time of hospital admission, 12.3 percent in the prophylactic anticoagulation group died within 30 days compared with 7.9 in the therapeutic anticoagulation group.
All groups had low bleeding rates, and there were no differences between the two therapeutic blood thinners for safety and efficacy.
NEJM
A Spanish team presents the first pharmacological treatment able to improve cardiac function in stiff-heart syndrome

The results of a study published in the New England Journal of Medicine (NEJM) promise to radically alter the prospects of patients with this disease. The study was led by Dr. Pablo García-Pavía, who heads the Inherited Cardiac Diseases Section at Hospital Universitario Puerta de Hierro and is a research scientist at the CNIC and within the Spanish cardiovascular research network (CIBERCV). Coinciding with the publication of the study, Dr. Pablo García-Pavía presented the results of the first clinical trial with an amyloid-removing drug for the treatment of cardiac amyloidosis.
The study represents a major advance in the treatment of the disease. Although currently available treatments effectively prevent the accumulation of more amyloid fibrils and delay disease progression, they do not directly remove any amyloid protein already deposited in the heart.
Current treatment options include transthyretin-stabilizing therapy and measures to control associated cardiovascular complications. The only intervention currently able to restore cardiac function in this disease is heart transplantation.
The initial results of the trial, which included 40 patients in France, the Netherlands, Germany, and Spain and was coordinated by Dr. García-Pavía, show that the new drug is safe and appears to reduce the amount of amyloid protein deposited in the heart.
Developed by the Swiss company Neurimmune, the new medication is an antibody that binds to transthyretin amyloid protein. The antibody was first isolated from memory B cells obtained from healthy elderly individuals.
In the study, the antibody was used to stimulate the patients’ own defense systems, resulting in the elimination of cardiac amyloid fibrils. The antibody was administered to patients intravenously in progressively increasing monthly doses over a 12-month period.
“Patients who received higher antibody doses seemed to show a greater reduction in amyloid deposits in the heart and greater improvements in a range of cardiac parameters,” said Dr. García-Pavía.
The NEJM article concludes that the phase I proof-of-concept study demonstrates the safety of this treatment in patients and supports further clinical trials of this antibody.
Dr. García-Pavía is a world-leading expert on transthyretin-related cardiac amyloidosis and is the leader of the European Society of Cardiology guidelines on the diagnosis and treatment of this disease, which are followed worldwide.
Nature
GLA, the fatty acid that makes the heart function properly after birth
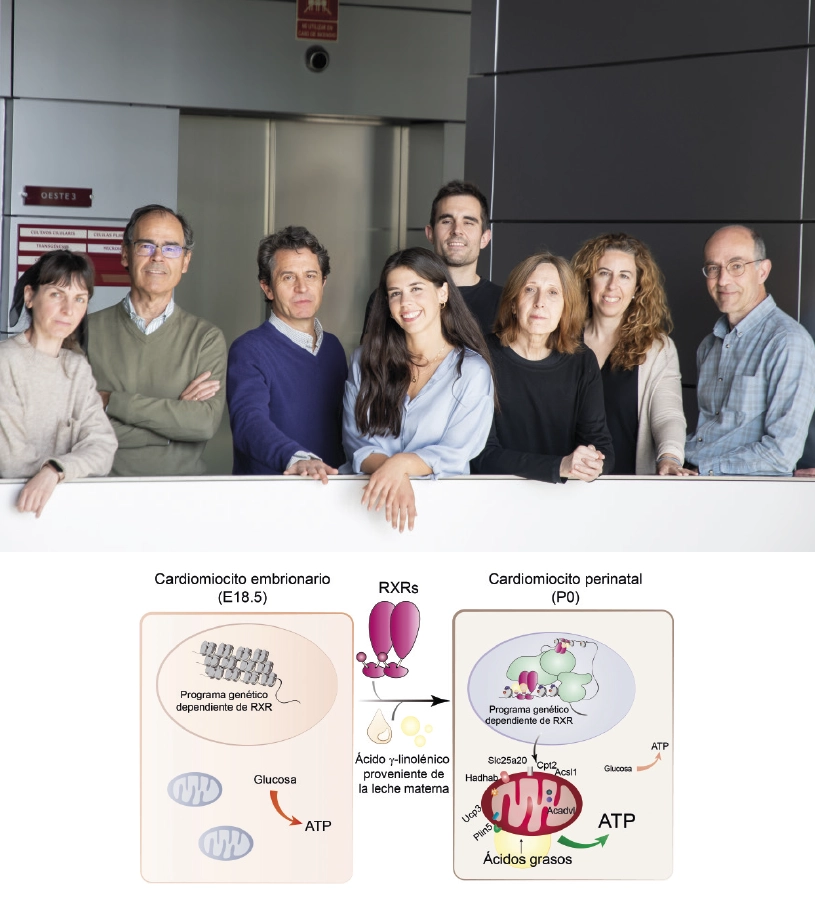
A study conducted in mice and led by researchers at the CNIC has revealed that maternal milk provides an essential signal that triggers the maturation of heart metabolism after birth, allowing the neonatal heart to function correctly and ensuring postnatal survival.
The study shows that the fatty acid gamma-linolenic acid (GLA), present in breast milk, binds to the retinoid X receptor (RXR) protein found in heart cells. RXR acts as a nutritional sensor of lipids and vitamin A derivatives, altering gene expression and influencing biological functions such as immunity, cell differentiation, and metabolism. Once activated by maternal GLA, RXR initiates genetic programs that equip mitochondria, the energy centers of the cell, with the enzymes and other proteins they need to start consuming lipids, the primary source of energy in the mature heart.
The results, published in Nature, could have significant therapeutic implications for cardiovascular disorders involving mitochondrial and metabolic dysfunction, as well as diseases related to alterations in postnatal developmental processes, explained study leader Dr. Mercedes Ricote, who heads the Nuclear Receptor Signaling group at the CNIC.
In a mouse model, the research team found that the absence of RXR in the heart or the lack of GLA in maternal milk prevented mitochondria in the hearts of newborn mice from producing energy correctly, leading to severe heart failure and death 24-48 hours after birth.
The heart of a newborn mammal must quickly produce energy to sustain cardiac contraction outside of the womb. To achieve this, cardiomyocytes, the contracting cells of the myocardium (the cardiac muscle), need to activate their mitochondria to use lipids as the energy source for the generation of ATP (adenosine triphosphate—the essential energy currency of the cell). Although this process is crucial for the survival of the organism, until now, little was known about the signals that trigger the physiological adaptation of the heart after birth.
“The need to maintain a constant and uninterrupted beat places an immense energy demand on the heart”, explained Dr. Ricote. “To meet their energy needs, cardiomyocytes maintain a tight control over the cellular pathways that produce energy. Any imbalance in these bioenergetic mechanisms can lead to the development of serious cardiovascular pathologies.”
For Dr. Ricote, part of the study’s novelty “lies in demonstrating that RXR plays a critical role in cardiac muscle, contrary to what was previously thought. This is an important conceptual advance in the field of nuclear receptors.”
According to first author Dr. Ana Paredes, the study presents a new framework for understanding the postnatal adaptations that occur in newborn mammals to meet the requirements of the extrauterine environment. “Birth is a physiological challenge for the newborn,” Dr. Paredes explained. “With this study, we show that maternal milk, besides its nutritional function, plays a signaling role by instructing cardiomyocytes that they need to activate their metabolism because they are no longer supported by maternal physiology.”
The research involved multidisciplinary approaches and advanced sequencing techniques, including key contributions from the CNIC teams led by Dr. José Antonio Enríquez, Dr. Fátima Sánchez-Cabo, and Dr. Jesús Vázquez.
Contributions from outside the CNIC included several Spanish centers: Centro Nacional de Biotecnología and Centro de Investigaciones Biológicas Margarita Salas, both belonging to the Consejo Superior de Investigaciones Científicas (CNB-CSIC, CIB-CSIC); Universidad Complutense de Madrid (UCM); Universidad de Barcelona (UB); Instituto de Biología Funcional y Genómica/Universidad de Salamanca (IBFG/USAL); CIBERCV, and CEMBIO/CEU San Pablo. International collaboration came from the Karolinska Institute (Sweden).
In conclusion, the study shows that the fatty acid GLA, found in breast milk, is the key signal that ensures correct cardiac function after birth. GLA activates the cell protein RXR, which then directs coordinated gene expression changes to ensure that cardiomyocyte mitochondria mature so that they can produce energy in the extrauterine environment.
The results open the way to treatments to modulate RXR activity in cardiomyocytes with specific drugs, including some that already have approval from the US Federal Drug Administration for cancer treatments. “Our study proposes RXR as a possible therapeutic target for neonatal heart disorders and systemic diseases triggered by metabolic errors,” concluded Dr. Ricote.
The study was supported by funding from the Spanish Ministry of Science and Innovation, Fundación La Marató de TV3, and the Community of Madrid regional government.
Nature Cardiovascular Research
Scientists identify how some angiogenic drugs used to treat cancer and heart disease cause vascular disease

Research by scientists at the CNIC has demonstrated that most of the molecular effects of medicines used to modulate the formation of new blood vessels (angiogenesis) in cardiovascular disorders and cancer are not responsible for the toxicity and vascular pathology triggered by these drugs. The study is published in Nature Cardiovascular Research.
Study leader Rui Benedito commented that “our results not only significantly increase our understanding of the biology of blood vessels, but will also help in the selection of the most effective and safe way to modulate angiogenesis in ischemic tissues or in cancer.”
The vascular system supplies oxygen and nutrients to the tissues and organs of the body. But the blood vessels do more than just conduct blood; they contribute actively to the physiology and homeostasis of all tissues and organs throughout life. Most blood vessels in the body are in an inactive state, which they maintain by expressing a large number of genes, including the genes of the signaling pathway mediated by Delta ligands and Notch receptors.
Several drugs have been developed in recent years that either block or induce angiogenesis in cardiovascular disorders or cancer.
A group of these compounds in clinical use inhibit different components of the Delta-Notch signaling pathway, which plays important roles in angiogenesis and in the maintenance of blood vessels in the inactive state. These compounds, by modulating the growth of blood vessels, efficiently block tumor growth. These compounds are also able to induce angiogenesis in ischemic tissues, thereby improving tissue regeneration and function.
However, these drugs can also cause vascular injury in organs with no previous disease, including the liver and heart, and this has reduced clinical interest in their use.
Until now, this vascular toxicity was thought to be due to the expression of genes that promote angiogenesis, leading the appearance of neoplasms or tumors in the affected blood vessels.
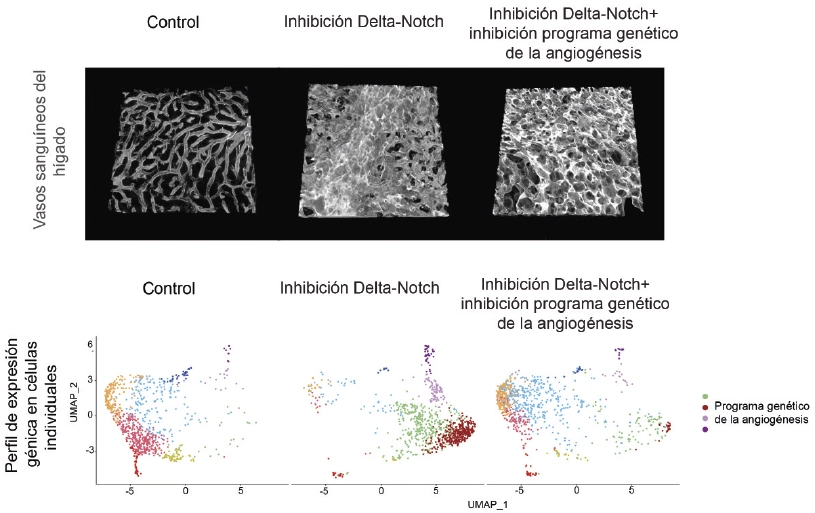
Thanks to the use of advanced genetic mouse models, high-resolution confocal microscopy, and single-cell sequencing and proteomics techniques, the team discovered that the vascular toxicity linked to these drugs is instead due to a change in the vascular architecture that impedes correct blood flow.
“Our study shows that the vascular pathology that can result from treatment with these drugs is unrelated to the expression of genes involved in angiogenesis or the appearance of neoplasms” said Rui Benedito.
The researchers showed that these changes happen even when they blocked cell activation and the expression of angiogenesis-related genes.
Therefore, explained Rui Benedito, “although the neoplasms and the expression of genes associated with angiogenesis are associated with the change in vascular architecture, they are not the cause of this change.”
First autor Macarena Fernández Chacón explained that “after analyzing different genes and drugs targeting blood vessels, we have found new ways to control pathological angiogenesis without significantly affecting vascular architecture in other organs, thus avoiding toxicity.”
The study was supported by the European Research Council (ERC) through Starting Grant AngioGenesHD and Consolidator Grant AngioUnrestUHD, the CNIC Severo Ochoa Intramural Program, the Spanish Ministry of Science and Innovation, and Fundación “la Caixa”.
European Heart Journal
Atherosclerosis accelerates aging

Atherosclerosis—the much-feared ‘hardening’ of our arteries—impacts our health long before the appearance of symptomatic cardiovascular disease. A new study by a team at the CNIC shows that atherosclerosis at subclinical stages accelerates the aging process.
The study was published in the European Heart Journal. Lead author and CNIC General Director Dr. Valentín Fuster emphasized that the results underline the benefits of reducing inflammation by adopting a healthy lifestyle (healthy diet, regular physical activity, etc.) or taking specific medication, such as colesterol-lowering statins “that can block, or at least slow, the transition from the subclinical phase of atherosclerosis to the appearance of severe cerebrovascular events, like myocardial infarction or stroke.”
The study shows that there is a strong association between the presence, extent, and progression of atherosclerosis at the subclinical level and accelerated epigenetic aging in otherwise healthy young individuals, said Dr. Enrique Lara Pezzi, an author on the study.
Epigenetic age is a measure of a person’s biological age (the functional age of their cells and tissues) based on the idea of the epigenetic clock. Epigenetic clocks use machine learning algorithms to predict a person’s biological age and life expectancy based on their level of DNA methylation, explained study first author Fátima Sánchez Cabo.
But sometimes, said Sánchez Cabo, this prediction does not correspond to a person’s chronological age (the time elapsed since birth), “so that someone’s epigenetic age can be older than their chronological age, whereas someone else might have an epigenetic age younger than their chronological age.”
Fortunately, unlike the germinal mutations we carry in our genome, “changes in DNA methylation are reversible, opening up the possibility of ‘slowing down’ our epigenetic aging,” assured Lara Pezzi.
The identification of an association between subclinical atherosclerosis and reduced life expectancy based on epigenetic clocks was possible thanks to a massive analysis of data from the PESA-CNIC-Santander study, which is led Dr. Valentín Fuster. Since 2010, the PESA-CNIC-Santander study has analyzed the progression of subclinical atherosclerosis in more than 4,000 Banco Santander employees aged 40 to 54 years at the start of the study and with no prior history of cardiovascular disease.
“The follow-up of this cohort constitutes one of the most important cardiovascular prevention studies in the world,” said Dr. Fuster.
The European Heart Journal study combines data on the progression of atherosclerosis obtained with advanced imaging techniques with detailed information on participants’ lifestyle and data from molecular omics studies.
“These molecular data allowed us to advance our knowledge of the causal mechanisms of subclinical atherosclerosis, as well as its clinical consequences, providing key information for a more personalized treatment of the disease in its early stages,” said Lara Pezzi.
Using transcriptomic and proteomic data, the study demonstrates that systemic inflammation triggered in individuals with a high burden of atherosclerotic plaques may be a key factor in accelerating their epigenetic aging.
The authors conclude that the study identifies a solid association between the presence, extent, and progression of subclinical atherosclerosis and accelerated epigenetic aging, mediated in part by low-grade chronic inflammation induced by inflammatory cytokines. The authors nevertheless recognize that further longitudinal studies are needed over a longer follow-up period and supported by more experimental data, in order to provide a more thorough characterization of the effect of atherosclerosis on health and life expectancy and to identify underlying mechanisms.
The PESA-CNIC-Santander study is cofunded by the CNIC and Banco Santander.
The study received funding from the Instituto de Salud Carlos III and the European Regional Development Fund (A way to build Europe) and the Spanish Ministry of Science and Innovation.













