Excellence in communication science
Leading journals publish CNIC science
eLife
CNIC scientists uncover opposing roles of p38 proteins in cardiac hypertrophy
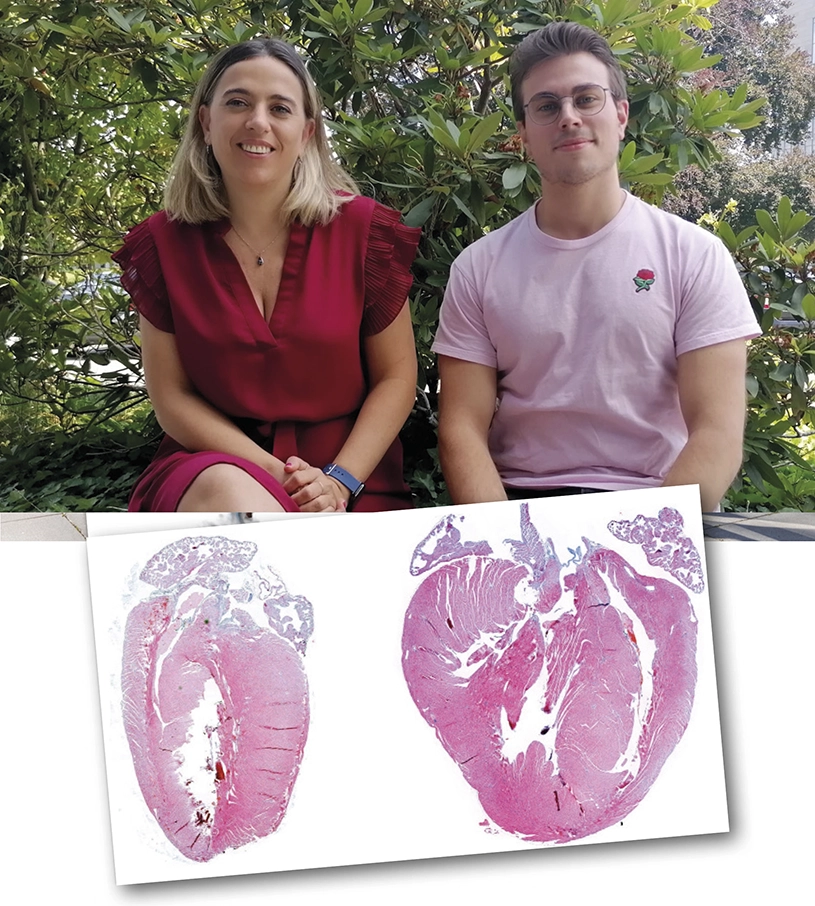
A study carried out by scientists at the CNIC and led by Dr. Guadalupe Sabio has identified a key role for the MKK3/6–p38γ/δ signaling pathway in the development of cardiac hypertrophy. The results, published in the journal eLife, suggest that inhibition of p38γ/δ could be a useful therapeutic strategy for diseases such as hypertrophic cardiomyopathy; however, this avenue remains unexplored because of the lack of specific inhibitors for these kinase enzymes. The study also shows the opposite effect upon inhibition of another member of this protein family, p38α, indicating that long-term clinical use of p38α inhibitors to treat chronic disease risks damage to the heart.
The heart has to satisfy this demand for blood not only under normal conditions, but also under conditions of stress. To achieve this, the heart has the capacity to increase in size, a process called hypertrophy. Cardiac hypertrophy is how the heart grows after birth and is also a normal response to physical exercise, explained first author Rafael Romero. But cardiac hypertrophy can also be triggered by high blood pressure and some genetic diseases.
The contractile cells of the heart, called cardiomyocytes, contain numerous molecular pathways whose activation promotes cardiac hypertrophy. “One of these is the p38 pathway, which is activated in response to stress stimuli,” explained Romero. The p38 proteins control a broad spectrum of processes, and their dysregulation has been linked to numerous diseases, making them promising pharmacological targets for clinical use. Nevertheless, specific inhibitors have been identified for only one of the p38 isoforms, p38α. As Dr. Sabio recognized, “the results of clinical trials to date have been disappointing, but other proteins of the pathway, such as p38γ, p38δ, or the upstream activator MKK6, are interesting possible alternative pharmacological targets.”
With this in mind, the team investigated the safety and potential long-term negative effects of inhibiting MKK6. Using mice genetically engineered to lack MKK6, the scientists showed that the absence of this protein reduced life expectancy. These mice developed cardiac hypertrophy when young and developed cardiac dysfunction as they got older. Using other mouse models, the researchers found that when MKK6 is absent the activation of p38α is significantly reduced. Nevertheless, inactivation of p38α promoted an unexpected activation of the other branch of the pathway, consisting of the proteins MKK3, p38γ, and p38δ. This activation induced another of the key pathways in the development of cardiac hypertrophy, the mTOR pathway.
The study recieved support from the following bodies: MINECO-FEDER, American Heart Association; EFSD/Lilly European Diabetes Research; Fundación AECC y Comunidad de Madrid IMMUNOTHERCAN-CM; Instituto Nacional de Corazón, Pulmón y Sangre; Fundación “la Caixa”; Fundación La Marató de TV3: Programa FP7 Marie Curie; EFSD Rising Star y JDC-2018-Incorporación (MIN/JDC1802).
The New England Journal of Medicine
The polypill reduces cardiovascular mortality by 33% in patients treated after myocardial infarction

The polypill developed by the CNIC and Ferrer, which includes three drugs (aspirin, an angiotensin-converting enzyme (ACE) inhibitor, and a statin), is effective at preventing secondary adverse cardiovascular events in people who have previously had a heart attack. The polypill reduces mortality from cardiovascular causes in this population by 33%. This is the finding of the SECURE study, coordinated by CNIC. The study results were published in The New England Journal of Medicine (NEJM).
SECURE included 2499 patients from 7 European countries (Spain, Italy, Germany, the Czech Republic, France, Poland, and Hungary) recovering after a myocardial infarction. The study participants were randomly assigned to receive standard therapy or the CNIC polypill.
The SECURE trial analyzed the incidence of four major cardiovascular events: death from cardiovascular causes, non-fatal myocardial infarction, non-fatal stroke, and emergency coronary revascularization (the restoration of blood flow through a blocked coronary artery). The study followed patients for an average of 3 years and produced conclusive results: patients taking the polypill had a 24% lower risk of these four events than patients taking the three drugs separately.
The standout finding of the study is the effect of the polypill on the key outcome of cardiovascular related death, which showed a relative reduction of 33%, from 71 patients in the group receiving standard treatment to just 48 in the polypill group.
The study also found that patients in the polypill group had a higher level of treatment adherence than those in the control group, thus confirming the findings of the earlier FOCUS2 study, also funded by the European Union.
The SECURE trial was funded by the European Union Horizon 2020 research and innovation program (trial identifier NCT02596126).
Journal of the American College of Cardiology
Scientists at the CNIC and Hospital Puerta de Hierro develop a tool to determine if dilated cardiomyopathy has a genetic origin

Scientists at the CNIC and Hospital Universitario Puerta de Hierro Majadahonda have developed a software application that predicts the likelihood that a case of dilated cardiomyopathy is caused by a genetic mutation. The research was carried out in collaboration with hospitals in Spain, Italy, and the Netherlands. The findings, published in the Journal of the American College of Cardiology (JACC), will allow physicians to adjust the treatment of dilated cardiomyopathy patients appropriately and to identify family members who have also inherited the disease. The software application is available online at www.madriddcmscore.com .
Dilated cardiomyopathy is the most frequent cause of heart failure in young people and the main indication for heart transplantation in the world. Nevertheless, in many places in the world dilated cardiomyopathy patients do not undergo routine genetic screening due to the considerable cost of this procedure, which gives a positive result in only 1 in every 3 patients
The new study was led by cardiologist Dr. Pablo García-Pavía of Hospital Puerta de Hierro, and who is also a research scientist at the CNIC and in the Spanish Cardiovascular Research Network (CIBERCV). The study analyzed the clinical characteristics, electrocardiograms, and echocardiography data of a group of 1015 dilated cardiomyopathy patients who underwent genetic screening at 20 Spanish hospitals. The results identified 5 parameters that were more frequent in patients in whom the disease was caused by a genetic mutation.
The combined scoring of these 5 parameters in a software application, called the Madrid Genotype Score, allows the classification of patients according to the likelihood that their disease has an origin in a heritable genetic mutation. The researcher team verified the predictive ability of the tool in an independent group of 1097 dilated cardiomyopathy patients from Italy and the Netherlands.
The software application has been made feely available to medical professionals via the website www.madriddcmscore.com.
Cellular and Molecular Sciences
Describing a new mechanism that links inflammation and pathological cardiovascular remodelling

Immune-inflammatory response contributes to the pathological remodelling of the arteries in different cardiovascular diseases. Research published by CMLS has shed new light on one of the mechanisms that links immune-inflammatory response to vascular disease, by describing the key role played by the early activation marker of lymphocytes CD69. The study, a collaboration of two CIBERCV groups at the Universidad Autónoma de Madrid (UAM) and IIBB-CSIC/IIB-Sant Pau, opens the way for new therapeutic strategies.
Antigen CD69, the early activation marker of lymphocytes, is a receptor that is induced after the leukocyte stimulation. Previous research by these teams identified the role of CD69 as an oxidised low density lipoprotein (oxLDL) receptor, a union giving an anti-inflammatory response that protects against atherosclerosis. Based on that previous work, this new study focuses on finding its possible role in the mechanisms that control inflammatory-immune response and the link with tissue remodelling in cardiovascular diseases.
By means of large-scale RNA sequencing (RNAseq) it was observed that the union of CD69 with oxLDL induces expression of PD-1 (a protein found in T lymphocytes that contributes to the control of immune response) and that this mechanism participates in regulation of immune response.
“This mechanism of PD-1 induction mediated by CD69 contributes to modulating inflammation and the cardiovascular remodelling that is produced as a consequence,” explain Francisco Sánchez Madrid and José Martínez González, heads of the CIBERCV group at the UAM and IIBB-CSIC respectively, and coordinators of this new research.
British Journal of Pharmacology
CNIC scientists identify a neuroprotective action of metoprolol after a stroke

A drug costing just €2 a shot can protect the brain during a stroke and greatly reduce long-term incapacity. Metoprolol, a beta-blocker in routine use in cardiology for more than 40 years, has now been shown to have a specific neuroprotective effect.
This is the finding of a study by scientists at the CNIC, Hospital Universitario Fundación Jiménez Díaz, and CIBERCV. The study was led by Dr. Borja Ibáñez and is published in the British Journal of Pharmacology.
The group led by Dr. Ibáñez—Clinical Research Director at the CNIC, cardiologist at Hospital Universitario Fundación Jiménez Díaz, and CIBERCV group leader—has demonstrated in a rat model that treatment with metoprolol protects the brain during a stroke and greatly reduces the severity of its long-term consequences. Rats that received intravenous metoprolol during the course of a stroke had less cerebral inflammation and neuronal death and better long-term improvement in neuromotor capacities.
The study received funding from the Carlos III Institute of Health; the European Regional Development Fund “A way to build Europe” program; the Comunidad de Madrid, with cofunding from the European structural and investment funds and the Spanish State Research Agency (Agencia Estatal de Investigación); and the European Research Council through the Horizon 2020 Research and Innovation Programme.
Nature Metabolism
European scientists discover how the body optimizes its respiratory capacity during exercise

A new study was carried out by an international team of researchers led by Prof. Johan Auwerx at the École polytechnique fédérale de Lausanne (EPFL) in Switzerland, working in partnership with scientists at the Spanish National Center for Cardiovascular Research (CNIC) and the Centro de Biología Molecular Severo Ochoa (CBMSO) in Madrid. The study confirms in humans the molecular mechanism through which mitochondria adapt their main energy-generating component—the electron transport chain (mtETC)—to optimize metabolism, cardiorespiratory function, and the capacity for exercise.
Dr. José Antonio Enríquez, an author on the study and head of the CNIC Functional Genetics of the Oxidative Phosphorilation System (GENOPHOS) group, explained that the mtETC is formed by four large multiprotein complexes, CI, CII, CIII, and CIV, which can combine in various structures called supercomplexes to perform different functions and adapt to local conditions.
The protein SCAF1 (also known as COX7A2L) is a key factor in the organization of the mETC. The study shows that SCAF1 expression is regulated in humans by population genetic variants. Study author Dr. Sara Cogliati explained that “the data demonstrate that there is a genetic variant of SCAF1 in humans that determines a higher expression in skeletal muscle and the heart.”
The study also shows that the frequency of the human SCAF1 variant differs between different geographical populations, “suggesting that mETC organization might contribute to the adaptation to different environmental conditions,” said Dr. Enríquez. The results of the study define for the first time in humans the fundamental role of the mETC in the adaptation of metabolism to varying energy demands.
The study received grant funding from the École polytechnique fédérale de Lausana; the European Research Council (ERC); the Swiss National Science Foundation (SNSF); the Marcel Levaillant Foundation; the Fondation suisse de recherche sur les maladies musculaires (FSRMM); the National Research Foundation of Korea through a Global Research Laboratory award; the HUNT Research Centre; the Ministerio de Ciencia, Innovación y Universidades; Agencia Estatal de Investigación; the European Regional Development Fund; the Spanish Biomedical Research Networking Center on Frailty Healthy Aging (CIBERFES-ISCiii); and the Human Frontier Science Program.
Nature Cardiovascular Research
CNIC scientists identify the cause of arrhythmias and sudden death in Andersen-Tawil syndrome type 1

Two research groups at the CNIC have discovered the cause of arrhythmias and sudden death in the rare disease Andersen-Tawil syndrome type 1 (ATS1), which is caused by mutations affecting potassium channels that regulate electrical activity and the intracellular calcium cycle in cardiac and skeletal muscle.
The teams led by Drs. José Jalife and Juan Antonio Bernal have discovered a previously unknown function of Kir2.1 channels, which control the essential electrical properties of excitable cells such as cardiac muscle, skeletal muscle, and neurons.
The Nature Cardiovascular Research study demonstrates that these distinct Kir2.1 microdomains are found in different species and different muscle cell types, indicating that they are involved in important and conserved cell functions.
In addition, said Dr. Bernal, the study reports “the generation and detailed characterization of a new mouse model of ATS1.”
The project was supported by the “La Caixa” Foundation, the Instituto de Salud Carlos III with cofunding from the European Regional Development Fund and European Social Fund, Fundació La Marató de TV3, and the European Union Horizon 2020 programme.
Journal of Clinical Investigation
CNIC scientists identify a factor that protects the heart against damage after a heart attack

A study carried out at the CNIC has identified a key factor that protects the heart after a heart attack. The study, led by Dr. Pilar Martín, who heads the Regulatory Molecules of Inflammatory Processes group at the CNIC, was published in the Journal of Clinical Investigation. The study shows that the expression of the receptor CD69 on regulatory T lymphocytes confers protection after a myocardial infarction by acting as a checkpoint for the exacerbated inflammation that causes medium-term cardiac injury.
The study involved the participation of researchers in the Spanish Biomedical Research Networking Center on Cardiovascular Diseases (CIBERCV), the group led by Dr. Francisco Sánchez-Madrid at the CNIC and Hospital Universitario de La Princesa, and the group led by Dr. José Martínez-González at the Instituto de Investigaciones Biomédicas de Barcelona (IIBB-CSIC) and the IIB-Sant Pau. The results show that the level of CD69 expression in peripheral blood can predict the development and progression of heart failure, which has severe consequences for heart function.
The authors discovered that the expression of CD69 on regulatory T lymphocytes increases in the first hours after the ischemic event. Through experiments with mouse models, the research team showed that the absence of CD69 leads to increases in inflammation, cardiac dysfunction, and the death rate after infarction.
In one of the key experiments of the study, the scientists injected CD69-expressing regulatory T cells into CD69-deficient mice after an infarction, finding that this treatment was able to make up for the deficiency of this molecule and thus decrease cardiac inflammation and improve survival.
The study also produced promising clinical findings through a follow-up analysis of myocardial infarction patients from two independent cohorts. This analysis revealed that the level of CD69 expression on peripheral blood cells predicts the development and progression of heart failure towards severe consequences for heart function.
The study received funding from the Ministerio de Ciencia e Innovación (MCIN) through the Instituto de Salud Carlos III (ISCIII) Fondo de Investigación Sanitaria, the Comunidad de Madrid, Fundació La Marató de TV3, the Spanish Biomedical Research Networking Center on Frailty and Healthy Aging (CIBERFES), the Human Frontier Science Program, Leducq Transatlantic Networks, Marie Skłodowska-grant, and University Teaching Staff Training Program of the Ministerio de Educación, Cultura y Deportes.
Cell Stem Cell
Researchers at CNIC, UPF, ICREA, CIBERNED and CIBERFES identify a mechanism that maintains mitochondria function in muscle stem cells and that can be stimulated in old age
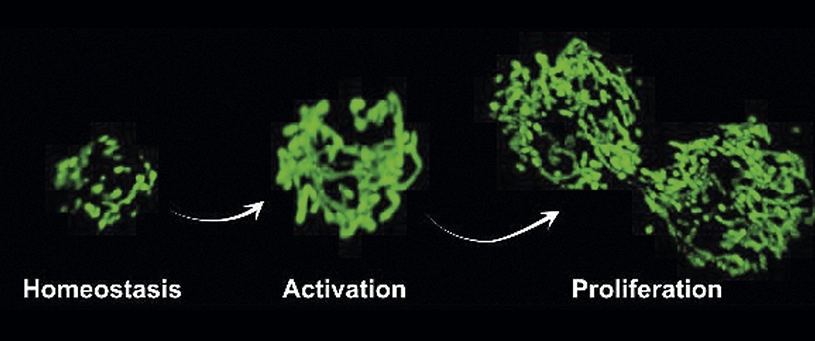
Researchers at the National Center for Cardiovascular Research (CNIC), Universidad Pompeu Fabra, ICREA, Centro de Investigación Biomédica de Enfermedades Neurodegenerativas (CIBERNED) and Centro de Investigación Biomédica en Red Fragilidad y Envejecimiento Saludable (CIBERFES) have identified a physiological mechanism that sustains the regenerative capacity of muscle stem cells, and that fails at old age. This failure can be overcome genetically and pharmacologically, hence restoring old stem cell regenerative functions.
Skeletal muscle regeneration depends on a muscle stem cell population (satellite cells) in a dormant or quiescent state, a situation that can be triggered by damage or stress to form new muscle fibres and expand in new stem cells.
The regenerative functions of these stem cells are known to decline with ageing. Dr. Pura Muñoz-Cánoves, senior investigator at the CNIC in Madrid, and ICREA professor at the MELIS Department at Pompeu Fabra University (UPF) in Barcelona, and Ciberned, and Dr. José Antonio Enríquez, senior investigator at CNIC and CIBERFES, and their colleagues, have found in experiments with mice that mitochondrial dynamics are required for tissue regeneration.
Mitochondrial fission facilitates muscle stem cell function via OXPHOS and mitochondrial autophagy (mitophagy) regulation. The researchers has shown that genetic loss of the mitochondria fission regulator DRP1 in muscle stem cells (or during aging) blunts their proliferation and regenerative capacity, whereas its reestablishment rescues these defects. According to the results presented in Cell Stem Cell, normalizing mitochondrial dynamics (or increasing OXPHOS and mitophagy) in aged muscle stem cells restores tissue regeneration.
This scientific study has also involved the collaboration of researchers at the University of Cordoba and the University of Padua (Italy). The study was funded in part by grants from the European Research Council (ERC), the Spanish Ministry of Science and Innovation, “La Caixa”-Health, Human Frontier Science Program and Leduq Foundation (LeduqRedox).
Circulation
Specific modifier genes determine the effect of mutations that cause non-compaction cardiomyopathy

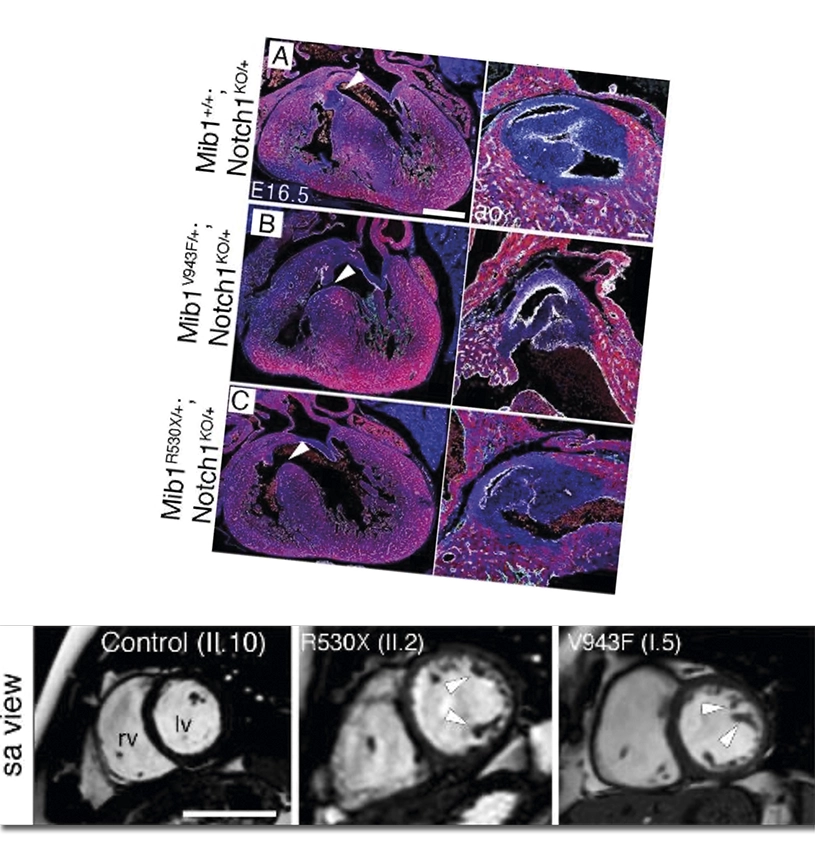
Non-compaction cardiomyopathy is a heart condition caused by defects that arise during fetal development and can have diverse health impacts in affected individuals, including sudden cardiac death. The Intercellular Signaling in Cardiovascular Development and Disease group at the CNIC previously reported that this disease can be caused by two distinct mutations in the Mindbomb1 gene (Mib1).
The same group, working with groups of CIBER de Enfermedades Cardiovasculares (CIBERCV) and CIBER de Bioingeniería, has now shown that the presence of one of these Mib1 mutations does not always lead to non-compaction cardiomyopathy. Instead, the outcome depends on the genetic context provided by mutations in other genes that “modify” the effect of the Mib1 mutations.
In a new article published in Circulation, the scientists describe how the context of mutations accompanying one supposedly “causal” mutation for non-compaction cardiomyopathy can condition both the severity and the appearance of different anomalies, so that the precise outcome depends on the patient’s specific combination of mutations.
The team led by Dr. José Luis de la Pompa used CRISPR-Cas9 molecular-scissor technology to insert the LVNC-causing mutations into the mouse genome.
These differences suggest that LVNC patients with an Mib1 mutation may additionally harbor “mutations in other genes that contribute to the severity and diversity of the observed defects.”
To uncover what was going on, the research team sought the help of the LVNC patients and their families, as well as partners from diverse centers, especially Dr. Juan Ramón Gimeno of Hospital Universitario Virgen de la Arrixaca. With these stakeholders on board, the scientists were able to sequence DNA from LVNC patients carrying Mib1 mutations and from their healthy family members.
These discoveries advance specific knowledge about LVNC and more generally reinforce the idea that congenital heart disease is not always monogenic, instead involving the intervention of several mutations through oligogenic inheritance.
The study received funding from the MCIN/AEI, CIBERCV, Fundación BBVA, the European Commission (H2020-HEALTH), the Medical Research Council, the British Heart Foundation, and Fundació La Marató de TV3.
Basic Research in Cardiology
A new therapeutic target for the prevention of heart failure due to aortic stenosis
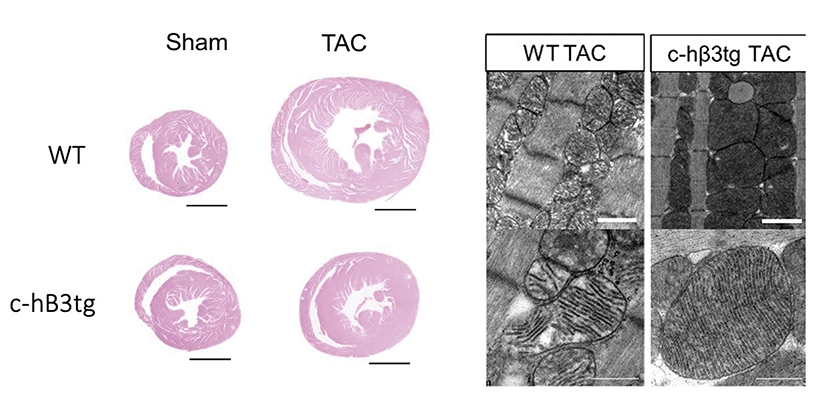
A study led by Dr. Borja Ibáñez, Clinical Research Director at the CNIC, shows that overexpression in cardiac muscle cells of beta-3 adrenergic receptor, a member of the beta adrenergic system, can prevent or even reverse heart failure in a mouse model of aortic stenosis, a condition that currently has few therapeutic options.
In the study, published in Basic Research in Cardiology, the CNIC team adopted an innovative gene-therapy approach to boost the expression of this receptor in the heart and thus reinforce its beneficial action.
Aortic stenosis is a progressive narrowing of the aortic valve, a ‘floodgate’ through which blood flows from the heart to the rest of the body. The condition is currently treated by replacing the damaged valve with a prosthesis.
While valve replacement technology has become much less invasive and successfully recovers valve function, the cardiac muscle, after years of being subject to stress, does not recover. Unfortunately, there is a lack of treatments able to improve cardiac muscle function and thereby alleviate heart failure resulting from a long history of aortic stenosis.
The study exploited the beneficial properties of stimulating the beta-3 adrenergic receptor, which is abundant in adipose tissue and the bladder but weakly expressed in the heart.
Through a collaboration with the CNIC Intercellular Signaling in Cardiovascular Development and Disease group, led by Dr. José Luis de la Pompa, transgenic mice were generated that overexpress the beta-3 adrenergic receptor in cardiomyocytes. When these mice were subjected to supravalvular aortic stenosis, they developed less cardiac hypertrophy and fibrosis than mice with normal levels of expression. The transgenic mice were also free of heart failure, and their hearts were metabolically more efficient and consumed less glucose.
Since the transgenic technology used to develop these mice is not applicable in patients, the investigators developed a gene therapy approach, whereby an innocuous virus was injected into mice to deliver the beta-3 adrenergic receptor gene specifically to cardiomyocytes, resulting in safe and efficient production of the receptor.
Working in partnership with the CNIC Viral Vectors Unit, the team designed an innocuous virus able to enter cardiomyocytes and drive elevated expression of beta-3 adrenergic receptor in the hearts of non-transgenic adult mice. When these mice were subjected to aortic stenosis, they were as equally protected against heart failure as transgenic mice overexpressing the receptor from before birth.
In a final test, the team injected the virus into non-transgenic mice with long-lasting aortic stenosis and established heart failure. In these mice, gene-therapy-induced overexpression of beta-3 adrenergic receptor recovered heart function, reduced cardiomyocyte hypertrophy, restored normal mitochondrial size and normal expression of mitochondrial fusion proteins in the heart, and increased animal survival.
The study has received funding from the Ministry of Science and Innovation of Spain (MICINN); European Commission; ERACVD Joint Translational Call 2016; European Regional Development Fund (ERDF); BBVA Foundation; CIBERCV, and TERCEL.
European Journal of Heart Failure
A promising drug treatment for patients with pulmonary hypertension associated with heart disease
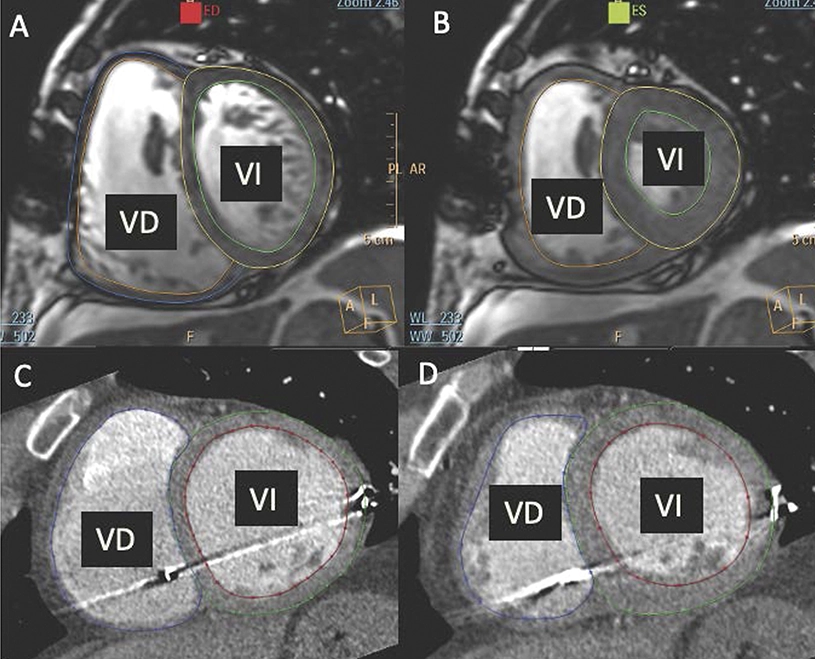
There is currently no specific treatment for pulmonary hypertension associated with heart disease, a highly prevalent condition with a poor prognosis. Now, a study from the Centro Nacional de Investigaciones Cardiovasculares (CNIC) and Hospital Clínic de Barcelona/IDIBAPS has shown that mirabegron, a drug that acts on the beta-3 adrenergic receptor, may have a beneficial effect on right ventricular function.
The SPHERE-HF study evaluated the beneficial potential of mirabegron in patients with pulmonary hypertension associated with heart disease. The results, published in the European Journal of Heart Failure do not show any improvement in pulmonary vascular resistance, the main study objective; however, they do show a beneficial effect on right ventricular function.
SPHERE-HF included 80 patients with heart disease and associated pulmonary hypertension. The patients were recruited at four tertiary hospitals—Hospital Sant Pau de Barcelona, Hospital Puerta de Hierro de Madrid, Hospital 12 de Octubre de Madrid, and Hospital Clínic de Barcelona—and were randomized to receive treatment with mirabegron or placebo over a 16-week period
All the patients were assessed by right heart catheterization to measure pulmonary pressures and by magnetic resonance imaging or cardiac computed tomography to evaluate right ventricular function. All cardiac images were evaluated by blinded experts at the CNIC.
These results suggest that beta-3 adrenergic receptor agonists have potential to improve right ventricular dysfunction, for which there is currently no treatment available suitable for chronic therapy.
The clinical trial was made possible through competitive funding from Fundació La Marató de TV3 and a collaborative partnership of five Spanish centers. The study also received funding from the European Commission (ERC-Consolidator Grant Agreement No. 819775), the Spanish Ministerio de Ciencia e Innovación, and the Comunidad de Madrid.
eLife
CNIC scientists identify the essential role of cell-surface “nanofolds” and “glue” in the mechanical response of cells

A study at the CNIC has revealed that subcellular structures called caveolae play an essential role in cell mechanics. The results suggest that impaired caveolar function could be involved in a variety of processes, including platelet aggregation, cardiovascular disease, fibrosis, and tumor formation. The study is published in eLife and was led by CNIC researchers Fidel-Nicolás Lolo and Miguel Ángel del Pozo. The results show that caveolae, by limiting abrupt changes in membrane tension, couple mechanical stress to the activity of integrins, thereby modifying the cellular mechanical responses.
The authors show that cells lacking this caveolar damping system have a dysfunctional mechanical response due to an anomalous increase in the expression of integrins at the cell membrane. Integrins are the main receptors for the extracellular matrix, the structure that “glues” cells to their local microenvironment.
Cells detect their microenvironment through tiny pulls and pushes that are transmitted through proteins called integrins, which are extracellular matrix receptors located on the cell surface. Dr. Lolo added that “integrins can switch between an inactive and an active state, with the active form being responsible for scanning the miroenvironment.”
Dr. Lolo explained that the relative amount of integrins at the cell surface is controlled by two main mechanisms, the regulation of plasma membrane tension and the dynamics of integrin recycling. Caveolae are small invaginations in the cell membrane, shaped like cups or folds. These organelles are found in many cell types and can regulate cell membrane tension by changing their shape.
An appropriate cellular mechanical response is critical for tissue homeostasis—the ability to respond to environmental challenges and maintain parameters within a physiological range. An inability to detect these environmental changes is a feature of disease processes including fibrosis, tumorigenesis, and cardiovascular disease and may play an important role in processes like platelet aggregation.
The study was supported by the European Union Horizon 2020 programme through a ITN Marie Skłodowska-Curie, the Spanish Ministerio de Ciencia e Innovación (including the Severo Ochoa Program), Fundación “la Caixa”, Asociación Española Contra el Cáncer, Fundació La Marató de TV3, and the Comunidad de Madrid.
Blood
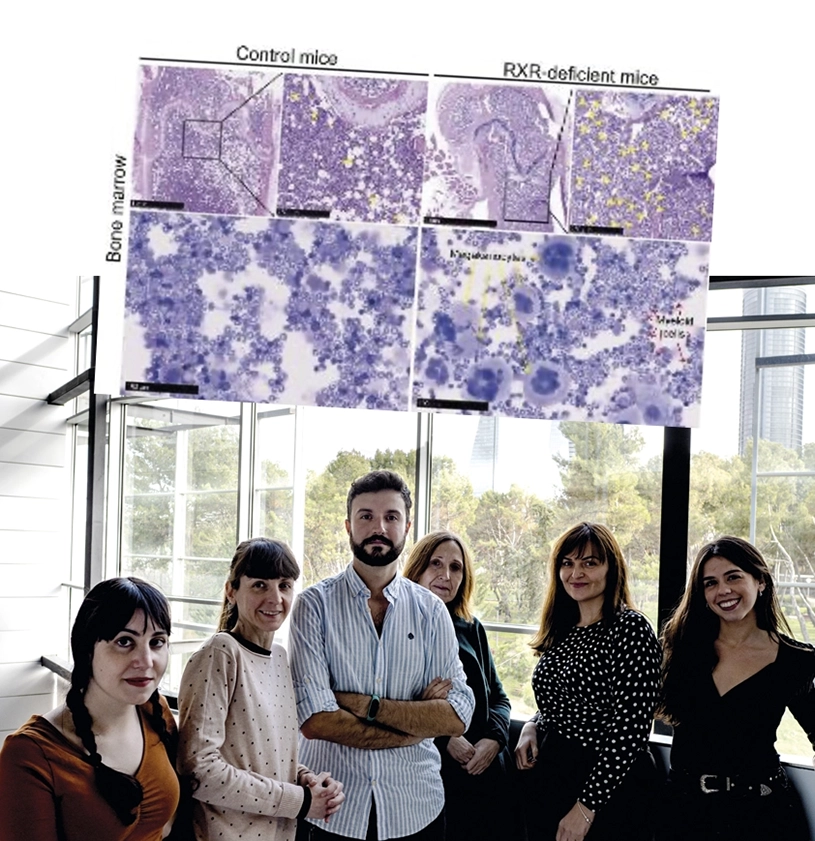
RXR, the cell protein that keeps blood stem cells young and fit
The cell protein retinoid X receptor (RXR) is a key factor in the maintenance of hematopoietic stem cells, the immature stem cells that give rise to all the blood cell lineages. RXR ensures that these cells remain youthful and fit, thereby reducing the risk of developing myeloproliferative syndromes as the body ages. The study, carried out by a team at the CNIC, demonstrates that the regulatory action of RXR on hematopoietic stem cells is essential for the maintenance of a balanced production of the spectrum of blood cell types throughout life.
The study findings, published in the journal Blood, could have therapeutic implications for conditions that feature excessive proliferation of myeloid blood cells, as is the case in some diseases of the blood and cardiovascular systems.
In the article, Dr. Mercedes Ricote’s group (CNIC) and the team led by Dr. José Cancelas (Cincinnati Children’s Hospital) demonstrate that the elimination of RXR from hematopoietic stem cells in mice triggers chronic expansion of a subgroup of cells skewed towards megakaryocytes (the progenitors of blood platelets) and the myeloid lineage, resulting in a deficit of the lymphoid lineage and the development of myeloproliferative syndrome as these mice age. The research shows that the excessive production of inflammatory myeloid cells in the RXR-deficient mice results in the invasion by these cells of multiple tissues, especially the lung, where they cause severe damage, leading to the premature death of these mice.
Collaboration with Dr. Sánchez-Cabo of the CNIC Bioinformatics Unit and Dr. Salomonis (Cincinnati Children’s Hospital) allowed the use of latest generation massive sequencing techniques and exhaustive analysis of DNA structure and gene expression in hematopoietic stem cells. The researchers emphasize the possibility of modulating RXR activity in hematopoietic stem cells through the use of drugs, some of them already used to treat cutaneous lymphomas.
The study was funded by grants from the Spanish Ministerio de Ciencia e Innovación (MICIN); Fundación La Marató de TV3; the Comunidad de Madrid, and the US National Institutes of Health.
Nature
Researchers characterize rare, damaged cells that block the functions of their neighbour healthy cells and identify ways to neutralize them and improve tissue regeneration
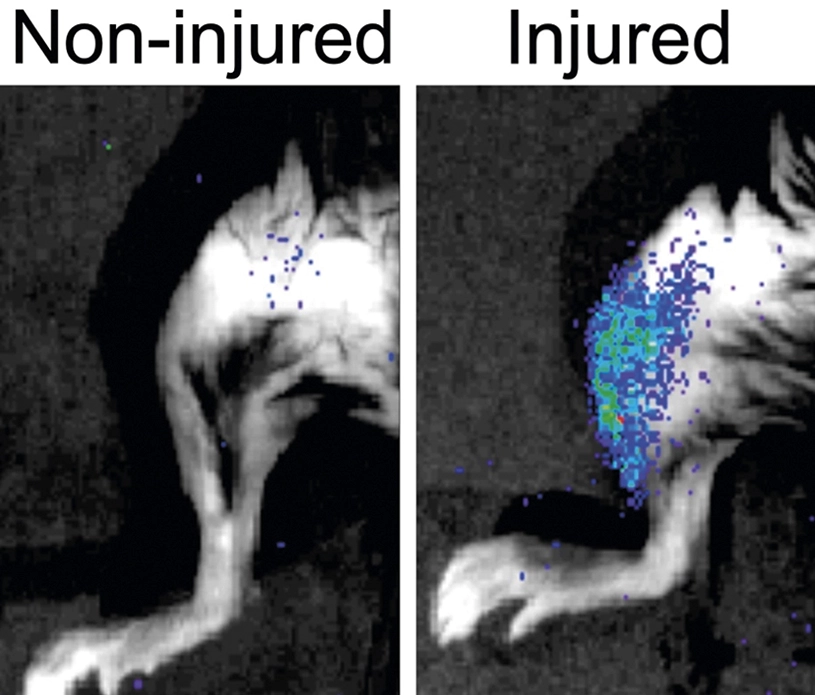
Researchers at the Universitat Pompeu Fabra (UPF), ICREA, CIBERNED, CNIC and Altos Labs, among other national and international collaborators, have characterized how damaged cells (senescent cells) that inevitably arise after injury negatively impact tissue regeneration, and how this mechanism operates actively in old age, but surprisingly also in young age. This negative action can be overcome genetically and pharmacologically, hence restoring stem cell regenerative functions.
Tissue regeneration depends on a population of stem cells and its neighbouring cells, a process whose efficacy declines with aging. The reasons of this decline remain largely unknown. Scientists have found in experiments with mice that senescent cells are new regulatory components of the muscle tissue regenerating niche that blunt muscle regeneration at all stages of life. Cellular senescence is a state of irreversible cell cycle arrest that often emerges after tissue damage and in age-related diseases.
In a study published in Nature, the team of researchers generated the first transcriptomic atlas of senescent cells of damaged skeletal muscle of mice of distinct ages (transcriptomic refers to everything related to RNA or the structures that transcript the information encoded originally inside a nucleus cell). Researchers found that senescent cells are widely heterogeneous, yet they display common traits, including the secretion of proinflammatory and profibrotic (that promotes an excess of fibrous connective tissue) factors. This secretion in turn, impacts the nearby stem cells and hampers their regenerative capacity, thus impairing muscle regeneration. So, it appears that what once was as a good protection tool now turns into a bad one.
Results showed that reducing the load of senescent cells (either through genetic or pharmacological treatments that induce death of these cells) improved the regeneration of aged muscles and, unexpectedly also, of young muscle. In addition to the biomedical benefits of targeting senescent cells, the new molecular information provided by the muscle senescent cell atlas could be likely transferred to understanding the function of senescence in other tissues whose senescent cells have either not been profiled at all or lack enough senescent cell numbers. The study has had the participation of the CNIC’s Genomic group led by Dr. Ana Dopazo.
The study was funded in part by grants from the European Research Council (ERC), the Spanish Ministry of Science and Innovation, “La Caixa” Foundation, AFM, MDA, MWRF, and DPP-Spain.
Nature Cell Biology
A study shows that cells possess two mechanisms to allow them to respond to different force ranges

A study carried out at the CNIC heralds a paradigm change in the field of mechanobiology. The study reveals that cells respond to forces of differing strength using two distinct mechanisms, one mediated by minute, cup-like invaginations on the cell surface called caveolae and the other by newly discovered large membrane depressions the study authors call dolines.
Study coordinator Miguel Ángel del Pozo, who heads the Mechanoadaptation and Caveolae Biology group at the CNIC, explained that the Nature Cell Biology study resolves controversies in this field. “Our results demonstrate that caveolae play an essential role in tissues that are subject to large mechanical forces (like skeletal muscle, heart muscle, blood vessels, and adipose tissue), whereas the newly identified dolines are important for the response to weak or medium-strength forces.”
Cells are constantly subjected to mechanical forces of different types and intensities originating in the local microenvironment, such as blood flow, the contraction and stretching of muscle, etc. To allow cells to respond and adapt their function to these stimuli, evolution has provided them with mechanisms for detecting different types of forces.
The most well-known structures with this capacity are caveolae (‘small caves’ in Latin). “These tiny invaginations in the plasma membrane [the outer envelope of the cell] are present on many types of cells and detect mechanical stimuli through changes in their physical shape. Caveolae flatten when cells swell or are stretched, rather like creases in a dress. But they reform and congregate when the cell membrane is relaxed,” said Miguel Ángel del Pozo.
These changes modulate biochemical signaling networks in the cell, making caveolae not only mechanical adaptors, but also transducers of mechanical information. However, before this study, it was unclear if this key function required the invagination of fully formed caveolae or if the individual components caveolin-1 and cavin-1 were sufficient by themselves.”
To investigate this question, the CNIC scientists set up a collaboration with biophysicist Universidad de Barcelona-IBEC, Pere Roca-Cusachs, to use magnetic tweezers to “elucidate which element is the mechanical sensor and which is the signal transducer,” explained Miguel Ángel del Pozo.
In addition to these experiments, the study collected many other biophysical parameters through partnerships with Spanish and international laboratories. The collected data demonstrated that cells expressing caveolin-1 but not cavin-1 sustained a mechanical response similar to that of cells expressing both proteins (and thus able to form caveolae).
Next, the researchers tried to determine the functional difference between caveolae and the isolated role of caveolin-1, “which was not an easy task,” comments Dr. Fidel Lolo. The caveolar response is on-off (switch-like), which only activates beyond a high force threshold and requires minutes. However, the new structures respond gradually, continuously, and immediately (within seconds) to lower and increasing force ranges.
Dr. Lolo suggested that “dolines may be especially important in cells like lymphocytes or neurons that don’t form caveolae but do express caveolin-1. These cells would thus be adapted to respond to more subtle microenvironmental forces in the tissues where they reside.”
The study was supported by the European Union Horizon 2020 Programme through a Marie Skłodowska-Curie ITN, the Spanish Ministry of Science and Innovation (including support through the Severo Ochoa Program), Fundación ”la Caixa” (AtheroConvergence project), Asociación Española Contra el Cáncer, Fundació La Marató de TV3, and the Community of Madrid regional government (‘Tec4Bio’ project).
















